Abstract
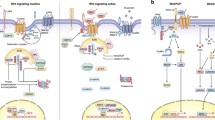
Wnt signaling is an evolutionarily conserved pathway that controls cell-to-cell interactions during embryogenesis. In adults, Wnt signaling plays a role in tissue homeostasis in almost every organ system. Aberrations within this pathway are implicated in a spectrum of human diseases. A variety of perturbations have been described in both solid and hematologic malignancies, lending way to Wnt signaling as a target for anti-cancer therapy. Of particular interest is the role of Wnt signaling in the development and maintenance of cancer stem cells, a rare population of cells that are able to maintain a tumor via self-renewal and thought to be more resistant to chemotherapy than bulk tumor cells. The ability to eradicate cancer stem cells may decrease the risk of cancer relapse and metastasis. A number of therapeutic agents specifically targeting the Wnt pathway have entered clinical trials, either as monotherapy or in combination with chemotherapy. We will provide an overview of agents that have been developed to target the Wnt pathways and a summary of pre-clinical and clinical trials.


Similar content being viewed by others
References
Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109.
Baker NE. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987;6(6):1765–73.
Loh KM, van Amerongen R, Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev Cell. 2016;38(6):643–55.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Katoh M. WNT/PCP signaling pathway and human cancer (review). Oncol Rep. 2005;14(6):1583–8.
Clevers H, Nusse R. Wnt/ β-catenin signaling and disease. Cell. 2012;149(6):1192–205.
Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13(12):767–79.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–50.
Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–51.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513–32.
Janda CY, et al. Structural basis of Wnt recognition by frizzled. Science. 2012;337(6090):59–64.
Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11(6):791–801.
Proffitt KD, Virshup DM. Precise regulation of porcupine activity is required for physiological Wnt signaling. J Biol Chem. 2012;287(41):34167–78.
Nile AH, Hannoush RN. Fatty acylation of Wnt proteins. Nat Chem Biol. 2016;12(2):60–9.
Langton PF, Kakugawa S, Vincent J-P. Making, exporting, and modulating Wnts. Trends Cell Biol. 2016;26(10):756–65.
Coombs GS, et al. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123(19):3357–67.
Liu J, et al. Targeting Wnt-driven cancer through the inhibition of porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110(50):20224–9.
Janku F, et al. Abstract C45: phase I study of WNT974, a first-in-class Porcupine inhibitor, in advanced solid tumors. Mol Cancer Ther. 2015;14(12 Supplement 2):C45.
Madan B, et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35(17):2197–207.
Bilic J, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316(5831):1619–22.
Kikuchi A, et al. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21–71.
Cruciat C-M, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5(3):a015081.
Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302(1):179–83.
Leyns L, et al. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88(6):747–56.
Lin K, et al. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci U S A. 1997;94(21):11196–200.
Li Y, et al. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22(21):3050–63.
Surmann-Schmitt C, et al. Wif-1 is expressed at cartilage-mesenchyme interfaces and impedes Wnt3a-mediated inhibition of chondrogenesis. J Cell Sci. 2009;122(20):3627–37.
Binnerts ME, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104(37):14700–5.
Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell. 2011;20(3):303–14.
Esteve P, et al. SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci. 2011;14(5):562–9.
Lee HX, et al. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124(1):147–59.
Aberle H, et al. β-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1997;16(13):3797–804.
Ikeda S, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 1998;17(5):1371–84.
Kishida S, et al. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J Biol Chem. 1998;273(18):10823–6.
Stamos JL, et al. Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. Elife. 2014;3:e01998.
Azzolin L, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–70.
Gammons MV, et al. Wnt signalosome assembly by DEP domain swapping of Dishevelled. Mol Cell. 2016;64(1):92–104.
Gammons MV, et al. Essential role of the Dishevelled DEP domain in a Wnt-dependent human-cell-based complementation assay. J Cell Sci. 2016;129(20):3892–902.
Zeng X, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135(2):367–75.
Fiedler M, et al. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin. Proc Natl Acad Sci U S A. 2011;108(5):1937–42.
Schwarz-Romond T, et al. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118(22):5269–77.
Wong H-C, et al. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of frizzled. Mol Cell. 2003;12(5):1251–60.
Jenny A, et al. Diego and Prickle regulate frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat Cell Biol. 2005;7(7):691–7.
Mlodzik M. Chapter five-the Dishevelled protein family: still rather a mystery after over 20 years of molecular studies. Curr Top Dev Biol. 2016;117:75–91.
Hecht A, et al. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19(8):1839–50.
Kahn M. Symmetric division versus asymmetric division: a tale of two coactivators. Future Med Chem. 2011;3(14):1745–63.
Chodaparambil JV, et al. Molecular functions of the TLE tetramerization domain in Wnt target gene repression. EMBO J. 2014;33(7):719–31.
Kumar A, et al. Zfp703 is a Wnt/β-catenin feedback suppressor targeting the β-catenin/Tcf1 complex. Mol Cell Biol. 2016;36(12):1793–802.
He X, et al. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131(8):1663–77.
Lu X, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430(6995):93–8.
Nishita M, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175(4):555–62.
Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31.
Habas R, Kato Y, He X. Wnt/frizzled activation of rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–54.
Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–40.
Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8(2):126–38.
Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134(4):647–58.
Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–46.
Kühl SJ, Kühl M. On the role of Wnt/β-catenin signaling in stem cells. Biochim Biophys Acta. 2013;1830(2):2297–306.
Clevers H, Loh KM, Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205).
Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7.
Ten Berge D, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13(9):1070–5.
O’Brien CA, Kreso A, Jamieson CHM. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16(12):3113–20.
Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15(4):338–44.
Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19(4):379–83.
Pinto D, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17(14):1709–13.
Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8.
Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–7.
Hermann PC, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23.
Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401.
Prince M, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–8.
Yang ZF, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–66.
Eramo A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–14.
Collins AT, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–51.
Curley MD, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27(12):2875–83.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84.
Hadnagy A, et al. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312(19):3701–10.
Chikazawa N, et al. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010;30(6):2041–8.
Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60.
Su L-K, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262(5140):1734–7.
Harada N, et al. Hepatocarcinogenesis in mice with beta-catenin and ha-Ras gene mutations. Cancer Res. 2004;64(1):48–54.
Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–70.
Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7.
Verras M, et al. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004;64(24):8860–6.
Qiang YW, et al. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106(5):1786–93.
Chien AJ, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106(4):1193–8.
Blanc E, et al. Low expression of Wnt-5a gene is associated with high-risk neuroblastoma. Oncogene. 2005;24(7):1277–83.
Jonsson M, et al. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62(2):409–16.
Kremenevskaja N, et al. Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene. 2005;24(13):2144–54.
Liang H, et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4(5):349–60.
Roman-Gomez J, et al. WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur J Cancer. 2007;43(18):2736–46.
Da Forno PD, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14(18):5825–32.
Kurayoshi M, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66(21):10439–48.
Ripka S, et al. WNT5A--target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28(6):1178–87.
Huang C-l, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor—an expression in non–small-cell lung cancer. J Clin Oncol. 2005;23(34):8765–73.
Mehdawi LM, et al. Non-canonical WNT5A signaling up-regulates the expression of the tumor suppressor 15-PGDH and induces differentiation of colon cancer cells. Mol Oncol. 2016;10(9):1415–29.
Bakker ER, et al. Wnt5a promotes human colon cancer cell migration and invasion but does not augment intestinal tumorigenesis in Apc1638N mice. Carcinogenesis. 2013;34(11):2629–38.
Wang Q, et al. Hypomethylation of WNT5A, CRIP1 and S100P in prostate cancer. Oncogene. 2007;26(45):6560–5.
Khaja ASS, et al. Elevated level of Wnt5a protein in localized prostate cancer tissue is associated with better outcome. PLoS One. 2011;6(10):e26539.
Khaja ASS, et al. Emphasizing the role of Wnt5a protein expression to predict favorable outcome after radical prostatectomy in patients with low-grade prostate cancer. Cancer Medicine. 2012;1(1):96–104.
Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115.
Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6(6):1017–28.
Satoh S, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24(3):245–50.
Liu W, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating β-catenin/TCF signalling. Nat Genet. 2000;26(2):146–7.
Cardona GM, et al. Identification of R-Spondin fusions in various types of human cancer. Cancer Res. 2014;74(19 Supplement):2408.
Madan B, Virshup DM. Targeting Wnts at the source--new mechanisms, new biomarkers, new drugs. Mol Cancer Ther. 2015;14(5):1087–94.
Suzuki H, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36(4):417–22.
Dahl E, et al. Frequent loss of SFRP1 expression in multiple human solid tumours: association with aberrant promoter methylation in renal cell carcinoma. Oncogene. 2007;26(38):5680–91.
Esteve P, Bovolenta P. The advantages and disadvantages of sfrp1 and sfrp2 expression in pathological events. Tohoku J Exp Med. 2010;221(1):11–7.
Joesting MS, et al. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65(22):10423–30.
Aguilera O, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25(29):4116–21.
Li S, et al. Dickkopf-1 is involved in invasive growth of esophageal cancer cells. J Mol Histol. 2011;42(6):491–8.
Sheng SL, et al. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009;55(9):1656–64.
Sato N, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010;70(13):5326–36.
Tian E, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–94.
Wissmann C, et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201(2):204–12.
Paluszczak J, et al. Frequent hypermethylation of WNT pathway genes in laryngeal squamous cell carcinomas. J Oral Pathol Med. 2014;43(9):652–7.
Nguyen DX, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138(1):51–62.
Medrek C, et al. Wnt-5a-CKI{alpha} signaling promotes {beta}-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells. J Biol Chem. 2009;284(16):10968–79.
Easwaran V, et al. Beta-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63(12):3145–53.
Chen MS, et al. Wnt/β-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120(3):468–77.
Chang HW, et al. Wnt signaling controls radiosensitivity via cyclooxygenase-2-mediated Ku expression in head and neck cancer. Int J Cancer. 2008;122(1):100–7.
Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–14.
Van Den Berg DJ, et al. Role of members of the Wnt Gene family in human hematopoiesis. Blood. 1998;92(9):3189–202.
Tickenbrock L, et al. Activation of Wnt signalling in acute myeloid leukemia by induction of frizzled-4. Int J Oncol. 2008;33(6):1215–21.
Valencia A, et al. Wnt signaling pathway is epigenetically regulated by methylation of Wnt antagonists in acute myeloid leukemia. Leukemia. 2009;23(9):1658–66.
Wang Y, et al. The Wnt/β-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–3.
Jamieson CH, et al. Granulocyte–macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351(7):657–67.
Nygren MK, et al. Wnt3A activates canonical Wnt signalling in acute lymphoblastic leukaemia (ALL) cells and inhibits the proliferation of B-ALL cell lines. Br J Haematol. 2007;136(3):400–13.
Ng O, et al. Deregulated WNT signaling in childhood T-cell acute lymphoblastic leukemia. Blood Cancer J. 2014;4(3):e192.
Walker MP, et al. FOXP1 potentiates Wnt/β-catenin signaling in diffuse large B-cell lymphoma. Sci Signal. 2015;8(362):ra12.
Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31(12):2670–84.
Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87(8):992–1005.
Brown WA, et al. Inhibition of beta-catenin translocation in rodent colorectal tumors: a novel explanation for the protective effect of nonsteroidal antiinflammatory drugs in colorectal cancer. Dig Dis Sci. 2001;46(11):2314–21.
Smith M-L, Hawcroft G, Hull M. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36(5):664–74.
Boon E, et al. Sulindac targets nuclear β-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90(1):224–9.
Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5(12):997–1014.
Castellone MD, et al. Prostaglandin E2 promotes Colon cancer cell growth through a Gs-Axin-ß-catenin signaling Axis. Science. 2005;310(5753):1504–10.
Liu Y, et al. Retinoic acid receptor beta mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol. 1996;16(3):1138–49.
Houle B, Rochette-Egly C, Bradley W. Tumor-suppressive effect of the retinoic acid receptor beta in human epidermoid lung cancer cells. Proc Natl Acad Sci U S A. 1993;90(3):985–9.
Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–21.
Easwaran V, et al. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9(23):1415–8.
Akhter J, et al. Vitamin D3 analog, EB1089, inhibits growth of subcutaneous xenografts of the human colon cancer cell line, LoVo, in a nude mouse model. Dis Colon rectum. 1997;40(3):317–21.
VanWeelden K, et al. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089 1. Endocrinology. 1998;139(4):2102–10.
Pálmer HG, et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol. 2001;154(2):369–88.
Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506.
Chen H-J, et al. The β-catenin/TCF complex as a novel target of resveratrol in the Wnt/β-catenin signaling pathway. Biochem Pharmacol. 2012;84(9):1143–53.
Badger TM, et al. Soy protein isolate and protection against cancer. J Am Coll Nutr. 2005;24(2):146S–9S.
Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125(3):777S–83S.
Zhang Y, et al. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/β-catenin signalling and reduces colon pre-neoplasia in rats. Br J Nutr. 2013;109(01):33–42.
Zhang Y, Chen H. Genistein attenuates WNT signaling by up-regulating sFRP2 in a human colon cancer cell line. Exp Biol Med. 2011;236(6):714–22.
Chen M, et al. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009;48(43):10267–74.
Osada T, et al. Antihelminth compound Niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011;71(12):4172–82.
Broome HE, et al. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk Res. 2011;35(10):1390–4.
Klein U, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194(11):1625–38.
Fukuda T, et al. Antisera induced by infusions of autologous ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci U S A. 2008;105(8):3047–52.
Yang J, et al. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS One. 2011;6(6):e21018.
Daneshmanesh A, et al. Monoclonal antibodies against ROR1 induce apoptosis of chronic lymphocytic leukemia (CLL) cells. Leukemia. 2012;26(6):1348–55.
Choi MY, et al. Pre-clinical specificity and safety of UC-961, a first-in-class monoclonal antibody targeting ROR1. Clin Lymphoma Myeloma Leukemia. 2015;15:S167–9.
Choi MY, et al. Immunotherapeutic Targeting of ROR1-Dependent, Non-Canonical Wnt5a-Signaling By Cirmtuzumab: A First-in-Human Phase I Trial for Patients with Intractable Chronic Lymphocytic Leukemia. Blood. 2016;128:3224.
Gurney A, et al. Wnt pathway inhibition via the targeting of frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109(29):11717–22.
Smith DC, et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. J Clin Oncol. 2013;31(15 Supplement):2540.
Von Hoff DD, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Zhang C, et al. Predictive biomarker identification for response to vantictumab (OMP-18R5; anti-Frizzled) using primary patient-derived human pancreatic tumor xenografts. Cancer Res. 2016;76(14 Supplement):3129.
Mita MM, et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with paclitaxel (P) in patients (pts) with 1st-to 3rd-line metastatic HER2-negative breast cancer (BC). J Clin Oncol. 2016;34(15 Supplement):2516.
Zhang C, et al. Predictive biomarker identification for response to vantictumab (OMP-18R5; anti-frizzled) by mining gene expression data of human breast cancer xenografts. Cancer Res. 2014;74(19 Supplement):2830.
Nagayama S, et al. Therapeutic potential of antibodies against FZD10, a cell-surface protein, for synovial sarcomas. Oncogene. 2005;24(41):6201–12.
Giraudet A.-L. et al. SYNFRIZZ-A phase Ia/Ib of a radiolabelled monoclonal AB for the treatment of relapsing synovial sarcoma. J Nucl Med. 2014;55(Supplement 1):223.
Pinzone JJ, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113(3):517–25.
Xiang XJ, et al. Differential expression of Dickkopf-1 among non-small cell lung cancer cells. Mol Med Rep. 2015;12(2):1935–40.
Edenfield WJ, et al. A phase 1 study evaluating the safety and efficacy of DKN-01, an investigational monoclonal antibody (Mab) in patients (pts) with advanced non-small cell lung cancer. J Clin Oncol. 2014;32(15 Supplement):8068.
Shepherd FA, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18(10):2095–103.
Edenfield WJ, et al. A phase 1 study evaluating the safety and efficacy of DKN-01, an investigational monoclonal antibody (Mab) in patients (pts) with advanced non-small cell lung cancer. J Clin Oncol. 2014;32(15 Supplement):8068.
Bendell JC, et al. Phase I study of DKN-01, an anti-DKK1 antibody, in combination with paclitaxel (pac) in patients (pts) with DKK1+ relapsed or refractory esophageal cancer (EC) or gastro-esophageal junction tumors (GEJ). J Clin Oncol. 2016;34(4 Supplement):111.
Fulciniti M, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–9.
Padmanabhan S, et al. A phase I/II study of BHQ880, a novel Osteoblat activating, anti-DKK1 human monoclonal antibody, in relapsed and refractory multiple myeloma (MM) patients treated with Zoledronic acid (Zol) and anti-myeloma therapy (MM Tx). Blood. 2009;114(22):750.
Munshi NC, et al. Early evidence of anabolic bone activity of BHQ880, a fully human anti-DKK1 neutralizing antibody: results of a phase 2 study in previously untreated patients with smoldering multiple myeloma at risk for progression. Blood. 2012;120(21):331.
Raje N, Roodman GD. Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res. 2011;17(6):1278–86.
Sonmez M, et al. Effect of pathologic fractures on survival in multiple myeloma patients: a case control study. J Exp Clin Cancer Res. 2008;27(1):11.
Hoey T. Development of FZD8-Fc (OMP-54F28), a Wnt signaling antagonist that inhibits tumor growth and reduces tumor initiating cell frequency. In: AACR Annual Meeting. 2013.
Jimeno A, et al. A first-in-human phase 1 study of anticancer stem cell agent OMP-54F28 (FZD8-Fc), decoy receptor for WNT ligands, in patients with advanced solid tumors. J Clin Oncol. 2014;32(15 Supplement):2505.
Weekes C, et al. Phase 1b study of WNT inhibitor ipafricept (IPA, decoy receptor for WNT ligands) with nab-paclitaxel (nab-P) and gemcitabine (G) in patients (pts) with previously untreated stage IV pancreatic cancer (PC). Ann Oncol. 2016;27(6 Supplement):367PD.
O’Cearbhaill RE, et al. Phase 1b of WNT inhibitor ipafricept (IPA, decoy receptor for WNT ligands) with carboplatin (C) and paclitaxel (P) in recurrent platinum-sensitive ovarian cancer (OC). J Clin Oncol. 2016;34(15 Supplement):2515.
Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5(2):100–7.
Agarwal P, et al. Inhibition of CML stem cell growth by targeting WNT signaling using a porcupine inhibitor. Blood. 2014;124(21):3130.
Säfholm A, et al. The Wnt-5a–derived Hexapeptide Foxy-5 inhibits breast cancer metastasis <em>in vivo</em> by targeting cell motility. Clin Cancer Res. 2008;14(20):6556–63.
Andersson T, et al. Abstract A116: targeting the Wnt-5a signaling pathway as a novel anti-metastatic therapy. Mol Cancer Ther. 2015;14(12 Supplement 2):A116.
Ma H, et al. Differential roles for the coactivators CBP and p300 on TCF//[beta]-catenin-mediated survivin gene expression. Oncogene. 2005;24(22):3619–31.
El-Khoueiry AB, et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31(15 Supplement):2501.
McWilliams RR, et al. A phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J Clin Oncol. 2015;33(15 Supplement):e15270.
Morishita EC, et al. Crystal structures of the armadillo repeat domain of adenomatous polyposis coli and its complex with the tyrosine-rich domain of Sam68. Structure. 2011;19(10):1496–508.
Cortes JE, et al. Phase 1 study of CWP232291 in relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). J Clin Oncol. 2015;33(15 Supplement):7044.
Yoon S-S, et al. Ongoing Phase 1a/1b Dose-Finding Study of CWP232291 (CWP291) in Relapsed or Refractory Multiple Myeloma (MM). Blood. 2016;128:4501.
Hood J, et al. Discovery of a small molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying treatment for knee osteoarthritis. Osteoarthr Cartil. 2016;24:S14–5.
Shou J, et al. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002;21(6):878–89.
Ohigashi T, et al. Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate. 2005;62(1):61–8.
Peng C, et al. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int J Gynecol Cancer. 2011;21(2):280–8.
Lee M, et al. Use of WNT inhibitors to augment therapeutic index of chemotherapy. Google Patents; 2010.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92.
Laine CM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med. 2013;368(19):1809–16.
Luke JJ, et al. Correlation of WNT/{beta}-catenin pathway activation with immune exclusion across most human cancers. J Clin Oncol. 2016;34(15 Supplement):3004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tabatabai, R., Linhares, Y., Bolos, D. et al. Targeting the Wnt Pathway in Cancer: A Review of Novel Therapeutics. Targ Oncol 12, 623–641 (2017). https://doi.org/10.1007/s11523-017-0507-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0507-4