Abstract
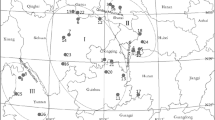
Cymbidium goeringii is a diploid and nonrewarding, bumblebee-pollinated species, which is distributed in China, Japan and Korea Peninsula. This species is now highly endangered due to the mass collection and forest clearance in China. In the present study, we investigated the distribution of genetic variation within and between eleven populations of Cymbidium goeringii in central China by using Inter-simple sequence repeats (ISSR) markers. Eleven primers produced a total of 127 clear and reproducible bands of which 112 were polymorphic. High genetic diversity was detected in Cymbidium goeringii for both population level (P = 63.1%; He = 0.194 5) and species level (P = 88.2%; He = 0.262 8). A higher level of genetic differentiation was detected among populations (G ST = 0.244 0, F ST = 0.220 7) with Nei’s G ST analysis and analysis of molecular variance (AMOVA), and no correlation was found between geographical and genetic distance. Genetic drift rather than gene flow played an important role in forming the present population structure of Cymbidium goeringii. Limited gene flow among populations and gene drift increase the extinction risk of local populations. Some conservation concerns are therefore discussed together with possible strategies for implementing in situ and ex situ conservation.
Similar content being viewed by others
References
Ackerman J D, Ward S (1999). Genetic variation in a widespread, epiphytic orchid: where is the evolutionary potential? Systematic Botany, 24: 282–291
Borba E L, Shepherd G J, Semir J (1999). Reproductive systems and crossing potential in three species of Bulbophyllum (Orchidaceae) occurring in Brazilian “campo rupestre” vegetation. Plant Systematics and Evolution, 217: 205–214
Bussell J D (1999). The distribution of random amplified polymorphic DNA (RAPD) diversity among populations of Isotoma petraea (Lobeliaceae). Molecular Ecology, 8: 775–789
Case M A (1993). High levels of allozyme variation within Cyperipedium calceolus (Orchidaceae) and low levels of divergence among its varieties. Systematic Botany, 18: 663–677
Case M A, Mlodozeniec H T, Wallace L E, Weldy T W (1998). Conservation genetics and taxonomic status of the rare Kentucky lady’s slipper: Cypripedium kentudkiense(Orchidaceae). American Journal of Botany, 85: 1779–1786
Chen S C, Tsi Z H (1998). The Orchids of China. Beijing: Chinese Forestry Publisher (in Chinese)
Chen S C, Tsi Z H, Lang K Y, Zhu G H (1999). Flora of China, Beijing: Science Press, 18: 171–178 (in Chinese)
Ciofi C, Beaumont M A, Swingland I R, Bruford M W (1999). Genetic divergence and units for conservation in the Komodo dragon Varanus komodoensis. Proceedings of the Royal Society B: Biological Science, 266: 2269–2274
Cozzolino S, Widmer A (2005). Orchid diversity: An evolutionary consequence of deception? Trends in Ecology and Evolution, 20: 487–494
D’amato F (1997). Role of somatic mutations in the evolution of higher plants. Caryologia, 50: 1–15
Doyle J J, Doyle J L (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin, 19: 11–15
Ehlers B K, Pedersen H (2000). Genetic variation in three species of Epipactis (Orchidaceae): Geographic scale and evolutionary inferences. Biological Journal of the Linnean Society, 69: 411–430
Ellstrand N C, Elam D R (1993). Population genetic consequences of small population size: Implications for plant conservation. Annual Review of Ecology and Systematics, 24: 217–242
Ferdy J, Loriot S, Sandmeier M, Lefranc M, Raquin C (2001). Inbreeding depression in a rare deceptive orchid. Canadian Journal of Botany, 79: 1,181–1,188
Frankham R, Ballou J D, Briscoe D A (2002). Introduction to Conservation Genetics. Cambridge: Cambridge University Press
Gustafsson S (2000). Patterns of genetic variation in Gymnadenia conopsea, the fragrant orchid. Molecular Ecology, 9: 1863–1872
Hamrick J L, Godt M J W (1989). Allozyme diversity in plant species. In: Brown A H D, Clegg M T, Kahler A L, Weir B S, eds. Plant Population Genetics, Breeding and Genetic Resources. Sunderland: Sinauer Associates, 43–63
Hamrick J L, Godt M J W (1996). Conservation genetics of endemic species. In: Avise J C, Hamrick J L, eds. Conservation Genetics: Case Histories from Nature. New York: Chapman and Hall, 281–304
Hutchison D W, Templeton A R (1999). Correlation of pairwise genetic and geographic distance measures: Inferring the relative influence of gene flow and drift on the distribution of genetic variability. Evolution, 53: 1898–1914
IUCN/SSC Orchid Specialist Group (1996). Orchids-Status Survey and Conservation Action Plan. Gland and Cambridge: IUCN
Kull T, Kindlmann P, Hutchings M J, Primack R B (2006). Conservation biology of orchids: Introduction to the special issue. Biological Conservation, 129: 1–3
Li A, Ge S (2006). Genetic variation and conservation of Changnienia amoena, an endangered orchid endemic to China. Plant Systematics and Evolution, 258: 251–260
Li A, Luo Y B, Ge S (2002). A preliminary study on conservation genetics of an endangered Orchid (Paphiopedilum micranthum) from southwestern China. Biochemical Genetics, 40: 195–201
Mantel N (1967). The detection of disease clustering and a generalized regression approach. Cancer Research, 27: 209–220
Nei M (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89: 583–590
Nybom H (2004). Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Molecular Ecology, 13: 1143–1155
Rohlf F J (2000). NTSYS-pc Version 2.1: Numerical Taxonomy and Multivariate Analysis System. Setauket: Exeter Software
Schaal B A, Hayworth D A, Olsen K M, Rauscher J T, Smith W A (1998). Phylogeographic studies in plants: Problems and prospects. Molecular Ecology, 7: 465–474
Schneider S, Roessli D, Excoffier L (2000). ARLEQUIN Version 2.0: A Software for Population Genetics Data Analysis. Geneva: Genetics and Biometry Laboratory, Department of Anthropology, University of Geneva
Shen L, Chen X Y, Li Y Y (2002). Glacial refugia and postglacial recolonization patterns of organisms. Acta Ecologica Sinica, 22: 1,983–1,990 (in Chinese)
Shi Y F, Cui Z J, Li J J (1989). Quaterany Glacials and Environmental Issues of Eastern China. Beijing: Science Press (in Chinese)
Slatkin M, Barton N H (1989). A comparison of three indirect methods for estimating average levels of gene flow. Evolution, 43: 1349–1368
Smith J L, Hunter K L, Hunter R B (2002). Genetic variation in the terrestrial orchid Tipularia discolor. Southeastern Naturalist, 1(1): 17–26
Sun M, Wong K C (2001). Genetic structure of three orchid species with contrasting breeding systems using RAPD and allozyme markers. American Journal of Botany, 88: 2180–2188
Tremblay R L, Ackerman J D (2001). Gene flow and effective population size in Lepanthes (Orchidaceae): A case for genetic drift. Biological Journal of the Linnean Society, 72: 47–62
Wang S, Xie Y (2004). China Species Red List. Beijing: Higher Education Press, 1: 433
Wolfe A D, Liston A (1998). Contributions of PCR-based methods to plant systematics and evolutionary biology. In: Soltis D E, Soltis P S, Doyle J J, eds. Plant Molecular Systematics II. Boston: Kluwer, 43-86
Wong K C, Sun M (1999). Reproductive biology and conservation genetics of Goodyera procera (Orchidaceae). American Journal of Botany, 86: 1,406–1,413
Yeh F C, Yang R C, Boyle T (1999). POPGENE Version l.31: Microsoft Window-based Freeware for Population Genetic Analysis. Edmonton: Department of Renewable Resources
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Biodiversity Science, 2006, 14(3): 250–257 [译自: 生物多样性]
Equally contributed authors
Rights and permissions
About this article
Cite this article
Yao, X., Gao, L. & Yang, B. Genetic diversity of wild Cymbidium goeringii (Orchidaceae) populations from Hubei based on Inter-simple sequence repeats analysis. Front. Biol. China 2, 419–424 (2007). https://doi.org/10.1007/s11515-007-0064-9
Issue Date:
DOI: https://doi.org/10.1007/s11515-007-0064-9