Abstract
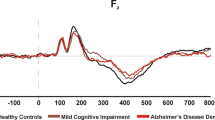
Mild cognitive impairment (MCI) is a stage between healthy aging and dementia. It is known that in this condition the connectivity patterns are altered in the resting state and during cognitive tasks, where an extra effort seems to be necessary to overcome cognitive decline. We aimed to determine the functional connectivity pattern required to deal with an internally directed cognitive state (IDICS) in healthy aging and MCI. This task differs from the most commonly employed ones in neurophysiology, since inhibition from external stimuli is needed, allowing the study of this control mechanism. To this end, magnetoencephalographic (MEG) signals were acquired from 32 healthy individuals and 38 MCI patients, both in resting state and while performing a subtraction task of two levels of difficulty. Functional connectivity was assessed with phase locking value (PLV) in five frequency bands. Compared to controls, MCIs showed higher PLV values in delta, theta, and gamma bands and an opposite pattern in alpha, beta, and gamma bands in resting state. These changes were associated with poorer neuropsychological performance. During the task, this group exhibited a hypersynchronization in delta, theta, beta, and gamma bands, which was also related to a lower cognitive performance, suggesting an abnormal functioning in this group. Contrary to controls, MCIs presented a lack of synchronization in the alpha band which may denote an inhibition deficit. Additionally, the magnitude of connectivity changes rose with the task difficulty in controls but not in MCIs, in line with the compensation-related utilization of neural circuits hypothesis (CRUNCH) model.




Similar content being viewed by others
References
Agrell B, Dehlin O (1998) The clock-drawing test. Age Ageing 27:399–403. doi:10.1093/ageing/27.3.399
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279. doi:10.1016/j.jalz.2011.03.008
Andrews-Hanna JR (2012) The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18:251–270. doi:10.1177/1073858411403316
Auer S, Reisberg B (1997) The GDS/FAST staging system. Int Psychogeriatr 9(Suppl 1):167–171
Aurtenetxe S, Castellanos NP, Moratti S et al (2013) Dysfunctional and compensatory duality in mild cognitive impairment during a continuous recognition memory task. Int J Psychophysiol 87:95–102. doi:10.1016/j.ijpsycho.2012.11.008
Bajo R, Maestú F, Nevado A et al (2010) Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J Alzheimers Dis 22:183–193. doi:10.3233/JAD-2010-100177
Bajo R, Castellanos NP, Cuesta P et al (2012) Differential patterns of connectivity in progressive mild cognitive impairment. Brain Connect 2:21–24. doi:10.1089/brain.2011.0069
Benedek M, Bergner S, Könen T et al (2011) EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia 49:3505–3511. doi:10.1016/j.neuropsychologia.2011.09.004
Benton A, Hamsher K (1989) Multilingual aplasia examination, 2nd edn. AJA Associates, Iowa City
Berendse H, Verbunt JP, Scheltens P et al (2000) Magnetoencephalographic analysis of cortical activity in Alzheimer’s disease: a pilot study. Clin Neurophysiol 111:604–612. doi:10.1016/S1388-2457(99)00309-0
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Buldú JM, Bajo R, Maestú F et al (2011) Reorganization of functional networks in mild cognitive impairment. PLoS One 6:e19584. doi:10.1371/journal.pone.0019584
Chételat G, Desgranges B, De La Sayette V et al (2002) Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport 13:1939–1943
Cirrito JR, Kang J-E, Lee J et al (2008) Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58:42–51. doi:10.1016/j.neuron.2008.02.003
Cooper NR, Croft RJ, Dominey SJJ et al (2003) Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol 47:65–74
De Haan W, Mott K, van Straaten ECW et al (2012) Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol 8:e1002582. doi:10.1371/journal.pcbi.1002582
Delbeuck X, Van der Linden M, Collette F (2003) Alzheimer’s disease as a disconnection syndrome? Neuropsychol Rev 13:79–92
Dimitriadis SI, Laskaris NA, Tsirka V et al (2010) What does delta band tell us about cognitive processes: a mental calculation study. Neurosci Lett 483:11–15. doi:10.1016/j.neulet.2010.07.034
Dubois B, Feldman HH, Jacova C et al (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746. doi:10.1016/S1474-4422(07)70178-3
Dubois B, Feldman HH, Jacova C et al (2010) Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 9:1118–1127. doi:10.1016/S1474-4422(10)70223-4
Fellgiebel A, Müller MJ, Wille P et al (2005) Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging 26:1193–1198. doi:10.1016/j.neurobiolaging.2004.11.006
Fernández A, Hornero R, Mayo A et al (2006) MEG spectral profile in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol 117:306–314. doi:10.1016/j.clinph.2005.10.017
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Friston KJ (2001) Brain function, nonlinear coupling, and neuronal transients. Neuroscientist 7:406–418
Fujie S, Namiki C, Nishi H et al (2008) The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 26:432–439. doi:10.1159/000165381
Garcia-Marin V, Blazquez-Llorca L, Rodriguez J-R et al (2009) Diminished perisomatic GABAergic terminals on cortical neurons adjacent to amyloid plaques. Front Neuroanat 3:28. doi:10.3389/neuro.05.028.2009
Gauthier S, Reisberg B, Zaudig M et al (2006) Mild cognitive impairment. Lancet 1262–1270
Gevins A, Smith ME, McEvoy L, Yu D (1997) High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb Cortex 7:374–385
Giannitrapani D (1971) Scanning mechanisms and the EEG. Electroencephalogr Clin Neurophysiol 30:139–146
Gómez C, Stam CJ, Hornero R et al (2009) Disturbed beta band functional connectivity in patients with mild cognitive impairment: an MEG study. IEEE Trans Biomed Eng 56:1683–1690. doi:10.1109/TBME.2009.2018454
Grundman M, Petersen RC, Ferris SH et al (2004) Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol 61:59–66. doi:10.1001/archneur.61.1.59
Haense C, Kalbe E, Herholz K et al (2012) Cholinergic system function and cognition in mild cognitive impairment. Neurobiol Aging 33:867–877. doi:10.1016/j.neurobiolaging.2010.08.015
Hämäläinen M, Hari R, Ilmoniemi RJ et al (1993) Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 65:413–497. doi:10.1103/RevModPhys.65.413
Harmony T, Fernández T, Silva J et al (1996) EEG delta activity: an indicator of attention to internal processing during performance of mental tasks. Int J Psychophysiol 24:161–171
Harmony T, Fernández T, Gersenowies J, Galán L, Fernández-Bouzas A, Aubert E, Díaz-Comas L (2004) Specific EEG frequencies signal general common cognitive processes as well as specific task processes in man. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology 53(3):207–16. doi:10.1016/j.ijpsycho.2004.04.006
He J, Farias S, Martinez O et al (2009) Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol 66:1393–1399. doi:10.1001/archneurol.2009.252
Ishii R, Shinosaki K, Ukai S et al (1999) Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport 10:675–679
Jelic V, Johansson S-E, Almkvist O et al (2000) Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol Aging 21:533–540. doi:10.1016/S0197-4580(00)00153-6
Jensen O, Mazaheri A (2010) Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4:186. doi:10.3389/fnhum.2010.00186
Jensen O, Gelfand J, Kounios J, Lisman JE (2002) Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex 12:877–882
Jeong J (2004) EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 115:1490–1505. doi:10.1016/j.clinph.2004.01.001
Jiang Z (2005) Study on EEG power and coherence in patients with mild cognitive impairment during working memory task. J Zhejiang Univ Sci B 6:1213–1219. doi:10.1631/jzus.2005.B1213
Jiang Z, Zheng L (2006) Inter- and intra-hemispheric EEG coherence in patients with mild cognitive impairment at rest and during working memory task. J Zhejiang Univ Sci B 7:357–364. doi:10.1631/jzus.2006.B0357
Jiang Z, Zheng L, Yu E-Y (2008) EEG coherence characteristics at rest and during a three-level working memory task in normal aging and mild cognitive impairment. Med Sci Monit 14:515–524
Kaplan E, Goodglass H, Weintraub S (1983) The Boston Naming Test. Lea and Febiger, Philadelphia
Klimesch W, Sauseng P, Hanslmayr S (2007) EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev 53:63–88. doi:10.1016/j.brainresrev.2006.06.003
Koenig T, Prichep L, Dierks T et al (2005) Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 26:165–171. doi:10.1016/j.neurobiolaging.2004.03.008
Krueger F, Landgraf S, van der Meer E et al (2011) Effective connectivity of the multiplication network: a functional MRI and multivariate Granger Causality Mapping study. Hum Brain Mapp 32:1419–1431. doi:10.1002/hbm.21119
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Leirer VM, Wienbruch C, Kolassa S et al (2011) Changes in cortical slow wave activity in healthy aging. Brain Imaging Behav 5:222–228. doi:10.1007/s11682-011-9126-3
Li X, Zhang Y, Feng L, Meng Q (2010) Early event-related potentials changes during simple mental calculation in Chinese older adults with mild cognitive impairment: a case-control study. Neurosci Lett 475:29–32. doi:10.1016/j.neulet.2010.03.038
Lobo A, Ezquerra J, Gómez Burgada F et al (1979) Cognocitive mini-test (a simple practical test to detect intellectual changes in medical patients). Actas Luso Esp Neurol Psiquiatr Cienc Afines 7:189–202
Locatelli T, Cursi M, Liberati D, Franceschi M, Comi G (1998) EEG coherence in Alzheimer’s disease. Electroen Clin Neuro 106(3):229–37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9743281
Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164:177–190. doi:10.1016/j.jneumeth.2007.03.024
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944
Micheloyannis S, Sakkalis V, Vourkas M et al (2005) Neural networks involved in mathematical thinking: evidence from linear and non-linear analysis of electroencephalographic activity. Neurosci Lett 373:212–217. doi:10.1016/j.neulet.2004.10.005
Molinuevo JL, Rami L (2013) Applying the IWG research criteria in clinical practice: feasibility and ethical issues. Med Clin North Am 97:477–484. doi:10.1016/j.mcna.2012.12.018
Morcom AM, Li J, Rugg MD (2007) Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex 17:2491–2506. doi:10.1093/cercor/bhl155
Moretti DV, Miniussi C, Frisoni GB et al (2007) Hippocampal atrophy and EEG markers in subjects with mild cognitive impairment. Clin Neurophysiol 118:2716–2729. doi:10.1016/j.clinph.2007.09.059
Moretti DV, Frisoni GB, Pievani M et al (2008) Cerebrovascular disease and hippocampal atrophy are differently linked to functional coupling of brain areas: an EEG coherence study in MCI subjects. J Alzheimers Dis 14:285–299
Mormann F, Lehnertz K, David P, Elger CE (2000) Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys D Nonlinear Phenom 144:358–369. doi:10.1016/S0167-2789(00)00087-7
Mufson EJ, Chen EY, Cochran EJ et al (1999) Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol 158:469–490. doi:10.1006/exnr.1999.7086
Norris G, Tate RL (2000) The Behavioural Assessment of the Dysexecutive Syndrome (BADS): ecological, concurrent and construct validity. Neuropsychol Rehabil 10:33–45. doi:10.1080/096020100389282
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Onton J, Delorme A, Makeig S (2005) Frontal midline EEG dynamics during working memory. Neuroimage 27:341–356. doi:10.1016/j.neuroimage.2005.04.014
Oostenveld R, Fries P, Maris E, Schoffelen J-M (2011) FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. doi:10.1155/2011/156869
Osipova D, Rantanen K, Ahveninen J et al (2006) Source estimation of spontaneous MEG oscillations in mild cognitive impairment. Neurosci Lett 405:57–61. doi:10.1016/j.neulet.2006.06.045
Palva S, Palva JM (2007) New vistas for alpha-frequency band oscillations. Trends Neurosci 30:150–158. doi:10.1016/j.tins.2007.02.001
Palva JM, Monto S, Kulashekhar S, Palva S (2010) Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci U S A 107:7580–7585. doi:10.1073/pnas.0913113107
Park JY, Lee KS, An SK et al (2012) Gamma oscillatory activity in relation to memory ability in older adults. Int J Psychophysiol 86:58–65. doi:10.1016/j.ijpsycho.2012.08.002
Parlato V, Lopez OL, Panisset M et al (1992) Mental calculation in mild Alzheimer’s disease: a pilot study. Int J Geriatr Psychiatry 7:599–602. doi:10.1002/gps.930070810
Peña-Casanova J (1990) Programa Integrado de Exploración Neuropsicológica- Test Barcelona. Protocolo, Masson
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194. doi:10.1111/j.1365-2796.2004.01388.x
Petersen RC, Doody R, Kurz A et al (2001) Current concepts in mild cognitive impairment. Arch Neurol 58:1985–1992
Petersen RC, Parisi JE, Dickson DW et al (2006) Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 63:665–672. doi:10.1001/archneur.63.5.665
Pfeffer RI, Kurosaki TT, Harrah CH et al (1982) Measurement of functional activities in older adults in the community. J Gerontol 37:323–329
Raichle ME, MacLeod AM, Snyder AZ et al (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682. doi:10.1073/pnas.98.2.676
Reisberg B, Ferris SH, de Leon MJ, Crook T (1982) The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 139:1136–1139
Reitan R (1958) Validity of the Trail Making test as an indicator of organic brain damage. Percept Mot Ski 8:271–276
Rémy F, Mirrashed F, Campbell B, Richter W (2004) Mental calculation impairment in Alzheimer’s disease : a functional magnetic resonance imaging study. Neurosci Lett 358:25–28. doi:10.1016/j.neulet.2003.12.122
Reuter-Lorenz PA, Cappell KA (2008) Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci 17:177–182. doi:10.1111/j.1467-8721.2008.00570.x
Rosen WG, Terry RD, Fuld PA et al (1980) Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 7:486–488. doi:10.1002/ana.410070516
Rossini PM, Del Percio C, Pasqualetti P et al (2006) Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 143:793–803. doi:10.1016/j.neuroscience.2006.08.049
Rypma B, Eldreth DA, Rebbechi D (2007) Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex 43:65–76
Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS et al (2010) Loss of “small-world” networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One 5:e13788. doi:10.1371/journal.pone.0013788
Sasaki K, Tsujimoto T, Nishikawa S et al (1996) Frontal mental theta wave recorded simultaneously with magnetoencephalography and electroencephalography. Neurosci Res 26:79–81
Sauseng P, Klimesch W, Doppelmayr M et al (2005) EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum Brain Mapp 26:148–155. doi:10.1002/hbm.20150
Stam CJ (2010) Use of magnetoencephalography (MEG) to study functional brain networks in neurodegenerative disorders. J Neurol Sci 289:128–134. doi:10.1016/j.jns.2009.08.028
Stam CJ, van Dijk BW (2002) Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Physica D: Nonlinear Phenomena 163(3–4):236–251. doi:10.1016/S0167-2789(01)00386-4
Stam CJ, van der Made Y, Pijnenburg YAL, Scheltens P (2003) EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand 108:90–96
Stam CJ, Jones BF, Manshanden I et al (2006) Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuroimage 32:1335–1344. doi:10.1016/j.neuroimage.2006.05.033
Tao H-Y, Tian X (2005) Coherence characteristics of gamma-band EEG during rest and cognitive task in MCI and AD. Conf Proc IEEE Eng Med Biol Soc 3:2747–2750. doi:10.1109/IEMBS.2005.1617040
Taulu S, Kajola M (2005) Presentation of electromagnetic multichannel data: the signal space separation method. J Appl Phys 97:124905. doi:10.1063/1.1935742
v d Pijnenburg YA, Made Y, van Cappellen van Walsum AM et al (2004) EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin Neurophysiol 115:1332–1339. doi:10.1016/j.clinph.2003.12.029
Warrington E, James M (1991) The visual object and space perception battery. Thames Valley Test Company, Bury St. Edmunds
Wechsler D (1987) Wechsler memory scale-revised (manual). The Psycho, San Antonio
Yener GG, Kurt P, Emek-Savaş DD et al (2013) Reduced visual event-related delta oscillatory responses in amnestic mild cognitive impairment. J Alzheimers Dis 37:759–767. doi:10.3233/JAD-130569
Yesavage JA, Brink TL, Rose TL et al (1982) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17:37–49
Zamarian L, Semenza C, Domahs F et al (2007a) Alzheimer’s disease and mild cognitive impairment: effects of shifting and interference in simple arithmetic. J Neurol Sci 263:79–88. doi:10.1016/j.jns.2007.06.005
Zamarian L, Stadelmann E, Nürk H-C et al (2007b) Effects of age and mild cognitive impairment on direct and indirect access to arithmetic knowledge. Neuropsychologia 45:1511–1521. doi:10.1016/j.neuropsychologia.2006.11.012
Zamrini E, Maestu F, Pekkonen E et al (2011) Magnetoencephalography as a putative biomarker for Alzheimer’s disease. Int J Alzheimers Dis 2011:280289. doi:10.4061/2011/280289
Zarahn E, Rakitin B, Abela D et al (2007) Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging 28:784–798. doi:10.1016/j.neurobiolaging.2006.03.002
Zhang H, Sachdev PS, Wen W et al (2012) Gray matter atrophy patterns of mild cognitive impairment subtypes. J Neurol Sci 315:26–32. doi:10.1016/j.jns.2011.12.011
Zheng L, Jiang Z, Yu E (2007) Alpha spectral power and coherence in the patients with mild cognitive impairment during a three-level working memory task. J Zhejiang Univ Sci B 8:584–592. doi:10.1631/jzus.2007.B0584
Acknowledgments
This study was supported by two projects PSI2009-14415-C03-01 and PSI2012-38375-C03-01 from the Spanish Ministry of Science and Economy, a predoctoral fellowship from the Ministry of Education (FPU AP-2008-00175), a PICATA predoctoral fellowship of the Moncloa Campus of International Excellence (UCM-UPM), a predoctoral fellowship from the Spanish Ministry of Science and Innovation (BES-2010-036469), and a predoctoral fellowship from the Basque Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
María Eugenia López and Pilar Garcés have contributed equally to this work.
About this article
Cite this article
López, M.E., Garcés, P., Cuesta, P. et al. Synchronization during an internally directed cognitive state in healthy aging and mild cognitive impairment: a MEG study. AGE 36, 1389–1406 (2014). https://doi.org/10.1007/s11357-014-9643-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-014-9643-2