Abstract
The occurrence of cyanobacteria in freshwaters attracts much attention due to its associated health threats and ecological implications. Yet data on the composition of cyanobacteria taxa and toxigenicity in some regions is still scarce. Here, we explored the occurrence of cyanobacteria and cyanotoxins in three locations in Ukraine (reservoir for Kasperivtsi Hydrothermal Power Plant and outflowing River Seret, and cooling pond of Khmelnytsky Atomic Power Plant) in summer 2017. Cyanobacteria were a dominant fraction at all stations. A number of potent-toxin producers were identified including Cylindrospermopsis raciborskii, Aphanizomenon gracile, Dolichospermum flos-aquae, and Planktothrix agardhii. Screening for the presence of dissolved and particulate content of microcystins (-LR, -YR, and -RR), cylindrospermopsin, and anatoxin-a yielded negative results. The studied waters displayed no toxicity in human platelets in vitro. Further toxicological and ecological studies are necessary to evaluate the potential presence of cyanotoxin producers in Ukraine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria from the Chroococcales, Nostocales, and Oscillatoriales orders are subject to ongoing interest due to their nearly global distribution, potent production of various toxins, wide range of ecological adaptations, and ability to expand to new habitats. Their occurrence in freshwaters is promoted by cultural eutrophication but can also be driven by climate changes. As predicted, the incidence of harmful cyanobacteria blooms in various regions will increase advocating a need to monitor toxigenic species in various regions (Paerl and Paul 2011; O’Neil et al. 2012).
Particular attention is being paid to species that are recognized as potent producers of cyclic hepatopeptides microcystins (MCs) that include Microcystis aeruginosa (Kütz.) Kützing, Planktothrix agardhii (Gomont) Anagnostidis & Komárek, and members of Nostoc, Anabaena/Dolichospermum, and Anabaenopsis (Bernard et al. 2017), as well as potent producers of the neurotoxic (homo)anatoxin-a (ANA-A) alkaloid that encompass species belonging to the Anabaena, Aphanizomenon, and Dolichospermum genera. Moreover, the occurrence of cytotoxic cylindrospermopsin (CYN) in European freshwater has been increasingly reported with strains of Aphanizomenon gracile (Lemm.) Lemmermann, A. klebahnii Elenkin ex Pechar, A. flos-aquae Ralfs ex Bornet et Flahault, A. ovalisporum Forti, and Oscillatoria sp. recognized as potent producers (Rzymski and Poniedziałek 2014).
In turn, Cylindrospermopsis raciborskii (Woloszyńska) Seenayya et Subba Raju, known as the main producer of CYN in sub-tropical and tropical areas, has never been documented to produce any known cyanotoxin in Europe although numerous studies have already demonstrated the toxicity of their exudates using in vivo and in vitro experimental models (Acs et al. 2013; Smutná et al. 2016; Rzymski et al. 2017a). Reported first in Lake Kastoria in Greece (Skuja 1937), it has systematically been found throughout the continent, although was rarely observed to form blooms (Budzyńska and Gołdyn 2017). With recent reports of its common occurrence in western parts of Poland, in a single lake in Lithuania (Kokocinski et al. 2017; Rzymski et al. 2017b) and Lake Nero in the Yaroslavl Region of Russia (Babanazarova et al. 2015), it appears that C. raciborskii continues to spread. Its distribution pattern and threats associated with its expansion in Eastern Europe are, however, still not sufficiently explored.
Here, we report the occurrence of C. raciborskii and other cyanobacteria belonging to the Chroococcales, Nostocales, and Oscillatoriales orders in three locations in Ukraine (water reservoir for Kasperivtsi Hydrothermal Power Plant and outflowing River Seret and cooling pond of Khmelnytsky Atomic Power Plant) along with analyses of cyanotoxins (CYN, MCs, and ANA-A), physicochemical parameters, and in vitro toxicity of water.
Material and methods
Study area and water sampling
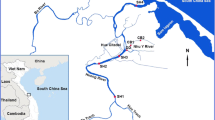
The samplings were carried out during August and September 2017 according to Environmental Protection Agency recommendations (Surface Water Sampling 2013). Surface water samples were collected at three sites (KHPP, RS, and KAPP) located in the Galicia-Volyn area, Western Ukraine (Fig. 1), transferred to propylene bottles, and transported to the laboratory for determination of chemical parameters, phytoplankton analyses, and toxicological studies. The Kasperivtsi hydroelectric power plant is situated near the Seret riverbed (48° 40′ N, 25° 51′ E). It is a small power plant with the installed capacity of 7.5 MW. Water discharged from the Kasperivtsi dammed water reservoir (KHPP site) flows through a turbine directly into the River Seret (RS site). The Kasperivtsi water reservoir (surface area 2.86 km2 and length 12 km) is a recreation area and the part of territory of National Nature Park “Dnister Canyon” where no industrial contamination is expected. The KAPP site, with a consistently higher water temperature, is located on the bank of the cooling pond of Khmelnytsky Atomic Power Plant (APP) in Netishyn (in a forestry area on the tributary of the River Goryn, 50° 21′ N, 26° 38′ E). There is no connection via water between these sites; the distance between the KHPP/RS and KAPP sites is about 300 km.
Phytoplankton analyses
The samples were preserved with Lugol solution and then analyzed within a month from sampling. Qualitative and quantitative phytoplankton analysis was performed in a Sedgwick-Rafter chamber of 0.5 mL volume, with the use of a light microscope Olympus BX60, according to standard methods (Wetzel and Likens 1991). Cyanobacterial and eukaryotic phytoplankton species were identified on the basis of the microscopic analysis of their morphological features, according to modern keys based on the polyphasic taxonomical approach (Komárek and Anagnostidis 1998; Komárek and Anagnostidis 2005; Komárek 2013).
Physicochemical water analyses
Water parameters were measured by routine analytical tests. pH was measured using an MI 150 tester with a combination electrode. In the field, water for chemical analyses was placed without preservation into polyethylene flasks and transported in coolers to the laboratory. The following water parameters were analyzed: nitrogen forms (N-NH4+ using the colorimetric method with Nessler reagent, N-NO2− colorimetric with Griess reagent, N-NO3− colorimetric with sodium salicylate) and total reactive phosphorus (TRP, using the molybdate method) (APHA 1995). Phenol concentration was evaluated using the 4-aminoantipyrine method (Dannis 1951). The chloride ion concentration was analyzed using the trimetric with silver nitrate method (EPA norm 9253 1994). Sulfate concentration in water samples was determined by indirect EDTA titration (Belle-Oudry 2008).
Concentrations of calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), manganese (Mn), iron (Fe), aluminum (Al), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), mercury (Hg), nickel (Ni), lead (Pb), and zinc (Zn) were quantified with the inductively coupled plasma optical emission spectrometer Agilent 5100 ICP-OES (Agilent, USA) after acidification of samples with nitric acid (Sigma-Aldrich, Germany) with conditions, wavelengths, and limit of detection (LOD) as described previously (Rzymski et al. 2017c). Calibration was performed using standard analytical solutions (Merck, Germany).
Cyanotoxin analyses
Water samples in volume from 200 to 500 mL were filtered through GF/C filters (Whatman, UK) to separate cyanobacterial cells from water and to determine dissolved and particulate concentration of cyanotoxins (microcystins (MCs), anatoxin-a (ATX), and cylindrospermopsin (CYN)) using the HPLC-DAD method.
MCs in the suspended material were extracted in 75% aqueous methanol and CYN and ATX were extracted in methanol. The samples were sonicated for 30 s in a Misonix (Farmingdale, NY, USA) ultrasonicator equipped with an ultrasonic probe (100 W, diameter 19 mm with “spike”) and the liquid processor XL. The extracts were then centrifuged twice at 11000×g for 10 min at 4 °C in an Eppendorf 5804 centrifuge (Hamburg, Germany). The supernatants were collected and evaporated in a SC110A Speedvac® Plus, ThermoSavant (Holbrook, NY, USA).
For dissolved MCs and ATX, samples of filtered water were concentrated on Baker (Deventer, Netherlands) C18 solid phase extraction (SPE) cartridges (sorbent mass 500 mg), conditioned earlier by 10 mL of methanol and water. Cyanotoxins were eluted from the C18 cartridges by 3 mL of 90% aqueous methanol containing 0.1% trifluoroacetic acid (TFA). The eluates were evaporated to dryness in a SC110A Speedvac® Plus, ThermoSavant (Holbrook, NY, USA). For dissolved CYN polygraphite carbon (PGC), solid phase extraction cartridges (sorbent mass 200 mg) and C18 solid phase extraction cartridges were used in series (C18 before PGC). For conditioning of the combined system, 10 mL of methanol containing 0.1% (v/v) TFA followed by 10 mL of water was used. CYN were eluted by 3 mL of 0.1% (v/v) TFA in methanol from PGC cartridges and then evaporated to dryness.
Before HPLC analysis, the samples were redissolved in 75% aqueous methanol for MC analyses and in water for CYN and ATX analyses and filtered through a Gelman GHP Acrodisc 13-mm syringe filter with a 0.45-μm GHP membrane and minispike outlet (East Hills, NY, USA). Chromatographic separation was performed using an Agilent (Waldbronn, Germany) 1100 series HPLC system consisting of a degasser, a quaternary pump, a column compartment thermostat set at 40 °C, and a diode array detector operated at 200–300 nm on a Merck (Darmstadt, Germany) Purospher STAR RP-18e column (55 mm × 4 mm I.D. with 3-μm particles) protected by a 4 mm × 4 mm guard column. The mobile phase consisted of water (solvent A) and acetonitrile (solvent B), both containing 0.05% trifluoroacetic acid. The flow rate was 1.0 mL/min with the following linear gradient program: 0 min, 1% B; 5 min, 7% B; 5.1 min, 70% B; 7 min, 70% B; 7.1 min, 1% B; stop time, 12 min for CYN and ATX analyses. The injection volume was 20 μL. CYN in the samples was identified by comparing the retention time and UV spectrum (200–300 nm) with an absorption maximum at 262 nm for CYN and at 227 nm for ATX. The flow rate for MC analyses was 1.0 mL min−1 with the following linear gradient program: 0 min, 25% B; 5 min, 70% B; 6 min, 70% B; 6.1 min, 25% B; stop time, 9 min. The injection volume was 20 μL. The contents of MC-LR, MC-YR, and MC-RR in the samples were analyzed by comparing the retention time and UV spectrum (200–300 nm with an absorption maximum at 238 nm).
In vitro toxicity assessment
The cytotoxicity of sampled water was assessed in an in vitro experimental model employing platelet-rich plasma (PRP) and assessing lactate dehydrogenase (LDH) leakage. This model was selected as cyanobacterial compounds can exhibit toxicity in platelets (Selheim et al. 2005). PRP was isolated by centrifugation (200×g, 12 min) from blood collected from five healthy donors at the Regional Centre of Blood and Blood Treatment in Poznan, Poland, according to accepted safeguard standards and legal requirements in Poland. One milliliter of PRP was incubated with 100 μL of filtered water samples for 1 h at 37 °C in darkness. Negative and positive controls were constituted of PRP incubated with phosphate-buffered saline and 10 μM of tert-butyl hydroperoxide (tBHP), respectively. The cytotoxicity was evaluated using Cytotoxicity Detection LDH Kit (Sigma-Aldrich, Germany) according to the manufacturer’s instructions. Briefly, following the incubation, all samples were centrifuged for 10 min at 1000 rpm and supernatants (100 μL) were transferred to a 96-well flat bottom microplate and mixed with a 100-μL reaction cocktail containing iodonitrotetrazolium chloride, sodium lactate, and diaphorase/NAD+ mixture. After incubation (30 min, 25 °C, darkness), the absorbance of each sample was read at 492 nm, and the cytotoxicity of the lake water sample was calculated according to the equation:
where control is a non-exposed sample and high control represents a non-exposed sample mixed with RIPA lysis buffer to evaluate the maximum releasable LDH activity for each sample.
Results and discussion
The physicochemical parameters of water during the sampling period are summarized in Table 1. The concentrations of toxic elements were low, indicating no industrial pollution although one should note that metal levels in water can be a subject to seasonal variations (Rzymski et al. 2014a). High sulfate concentrations, exceeding threefold the maximum allowance level set for drinking water in the European Union (Directive 98/83/EC 1998), were noted at all sampling stations in August. Periodically increased levels of this parameter are observed in surface waters of this region and can be, at least partially, explained by high sulfate concentration in soil (Choban and Winkler 2010; Peryt et al. 2012). In general, the studied waters were eutrophic as indicated by inorganic nitrogen and phosphate concentrations. This was also evident from phytoplankton analyses—cyanobacteria were a dominant fraction in all the studied samples in both August and September, with abundance ranging from 78% to as much as 98% depending on period and studied site (Fig. 2). A share of each identified cyanobacterial species in the total phytoplankton and cyanobacteria community is given in Table 2. The dominant taxa at KHPP and RS sites included Pseudanabaena sp. and Planktothrix agardhii (Fig. 3c), whereas P. agardhii and Planktolyngbya limnetica dominated at the KAPP site. M. aeruginosa was identified only at the KAPP site and only in September with a 17% share in total phytoplankton. C. raciborskii (Fig. 3a) and Sphaerospermopsis aphanizomenoides (Fig. 3d) were identified at the KHPP and RS sites with their share in total phytoplankton reaching a maximum of 8.5 and 6.3%, respectively. A. gracile was identified at all studied sites but at very low abundances (Table 2).
This report highlights the presence of number of potent toxin-producing cyanobacteria in man-made water reservoirs in Ukraine. Although species such as C. raciborskii and M. aeruginosa have been reported earlier in Ukraine (Novoselova and Protasov 2016), the data on their potential toxicity is scarce and focused only on MCs. As provided by Belykh et al. (2013), screening of mcy genes in the freshwater of Kiev regions yielded positive results in the majority of studied samples, although exact producers were not identified. Previous phytoplankton screening conducted in 2005 in the Kasperivtsi Hydrothermal Power Plant reservoir and outflowing River Seret did not identify C. raciborskii, P. agardhii, or A. gracile (Shcherbak and Bondarenko 2005). Therefore, the findings of the present study indicate the potential expansion of these cyanobacteria in this region and highlight that their further dispersion through a river system is plausible.
Our study identified two main potent producers of MCs: M. aeruginosa and P. agardhii, but even though the latter reaches a relatively high abundance, the dissolved and particulate content of all three studied MC analogues was found below detection limits. However, the occurrence of non-MC-producing strains of both species is a common phenomenon (Yéprémian et al. 2007; Park et al. 2018), and previous studies have indicated that the temporal and spatial distribution of toxic and non-toxic genotypes can be attributed to a number of factors including resource competition (Briand et al. 2008; Lei et al. 2015; Suominen et al. 2017).
Potential ANA-A-producing cyanobacteria identified in this study included Cuspidothrix issatschenkoi (Usačev) Rajaniemi et al. (Ballot et al. 2010) and Dolichospermum flos-aquae (Lyngbye) Wacklin, Hoffmann et Komárek (Osswald et al. 2009), although this neurotoxin was not detected. Moreover, no dissolved or particulate CYN was found in the studied samples, excluding its production by the identified strain of A. gracile (Fig. 1b), a species previously demonstrated as a major CYN producer in Poland (Kokociński et al. 2013). Our findings also support a preliminary view that Ukrainian strains of C. raciborskii are unable to produce CYN. The abundance of this species in the studied waters was relatively low, in line with previous observations in Polish lakes where C. raciborskii biomass, even during cyanobacterial blooms, was usually not high, and maximally accounted for 40% of total phytoplankton biomass (Kokocinski et al. 2017). However, there was a single reported case when C. raciborskii dominated the phytoplankton of a shallow reservoir in Poland after a particularly hot and sunny early summer (Budzyńska and Gołdyn 2017). Moreover, long-term phytoplankton analysis in Poland showed the ability of C. raciborskii to outcompete a native bloom-forming cyanobacterial species Planktothrix agardhii (Kokociński et al. 2010). It was also experimentally evidenced that the Polish strain of C. raciborskii can outcompete M. aeruginosa even at relatively low initial biomass (Rzymski et al. 2014b). Therefore, the dynamic of C. raciborskii occurrence in Ukraine surface waters requires further monitoring.
Additionally, the collected waters were screened for their toxic properties in human PRP in vitro. The employed model is convenient as it allows a study of reactions in the presence of extracellular components while platelets were previously reported to respond to toxic compounds during short-time exposure and release LDH during their lysis (Kim et al. 2009). It was found that the collected waters did not display a significant in vitro toxicity in human platelets (< 3% cytotoxicity) as evidenced using an LDH assay (Fig. 4). This rather excludes the presence of significant concentrations of some other toxic compounds in the collected water.
The cytotoxicity of filtered water samples in human platelet-rich plasma assessed by means of lactate dehydrogenase leakage assay and compared to that exerted by tert-butyl hydroperoxide (t-BHP; positive control). Asterisk indicates statistically significant difference with control (p < 0.05; Student t test)
In summary, this study explored the presence of cyanobacteria in the water reservoirs of power plants in Ukraine demonstrating that this group dominated phytoplankton during summer. Although potent producers of CYN, ANA-A, and MCs were identified, the intra- and extracellular presence of these cyanotoxins was not confirmed. Further toxicological and ecological studies, including molecular investigations on isolated strains, are required to evaluate the presence of potent cyanotoxin producers and associated health threats in this geographical region.
References
Acs A, Kovács AW, Csepregi JZ, Törő N, Kiss G, Győri J, Vehovszky A, Kováts N, Farkas A (2013) The ecotoxicological evaluation of Cylindrospermopsis raciborskii from Lake Balaton (Hungary) employing a battery of bioassays and chemical screening. Toxicon 70:98–106
APHA American Public Health Association (1995) Standard methods for the examination of water and waste water. APHA-AWWA-WEF, New York
Babanazarova OV, Sidelev SI, Fastner J (2015) Northern expansion of Cylindrospermopsis raciborskii (Nostocales, Cyanoprokaryota) observed in shallow highly eutrophic Lake Nero (Russia). Int J Algae 17:131–141
Ballot A, Fastner J, Lentz M, Wiedner C (2010) First report of anatoxin-a producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany. Toxicon 56:964–971
Belle-Oudry D (2008) Quantitative analysis of sulfate in water by indirect EDTA titration. J Chem Educ 85:1269
Belykh OI, Gladkikh AS, Sorokovikova EG, Tikhonova IV, Potapov SA, Fedorova GA (2013) Microcystin-producing cyanobacteria in water reservoirs of Russia, Belarus and Ukraine. Chem Sustain Dev 21:347–361
Bernard C, Ballot A, Thomazeau S, Maloufi S, Furey A, Mankiewicz-Boczek J, Pawlik-Skowronska B, Capelli C, Salmaso N (2017) Appendix 2. Cyanobacteria associated with the production of cyanotoxins. In: Meriluoto J, Codd GA SL (eds) Handbook on cyanobacterial monitoring and cyanotoxin analysis. Wiley, New York, pp 503–527
Briand E, Yéprémian C, Humbert JF, Quiblier C (2008) Competition between microcystin- and non-microcystin-producing Planktothrix agardhii (cyanobacteria) strains under different environmental conditions. Environ Microbiol 10:3337–3348
Budzyńska A, Gołdyn R (2017) Domination of invasive Nostocales (Cyanoprokaryota) at 52oN latitude. Phycol Res 65:322–332
Choban A, Winkler I (2010) Problems of regulation of anthropogenic load on the water objects with various dilution ratio in Ukraine. In: Hlavinek P, Winkler I, Marsalek J, Mahrikova I (eds) Advanced water supply and wastewater treatment: a road to safer society and environment. Springer, Dordrecht
Dannis M (1951) Determination of phenols by the amino-antipyrine method. Sewage Ind Waste 23:1516–1522
Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption
EPA Method 9253 (1994) Chloride (titrimetric, silver nitrate). https://www.epa.gov/sites/production/files/2015-12/documents/9253.pdf. Accessed 21.01.18
Kim EJ, Lim KM, Kim KY, Bae ON, Noh JY, Chung SM, Shin S, Yun YP, Chung JH (2009) Doxorubicin-induced platelet cytotoxicity: a new contributory factor for doxorubicin-mediated thrombocytopenia. J Thromb Haemost 7:1172–1183
Kokociński M, Stefaniak K, Mankiewicz-Boczek J, Izydorczyk K, Soininen J (2010) The ecology of the invasive cyanobacterium Cylindrospermopsis raciborskiii (Nostocales, Cyanophyta) in two hypereutrophic lakes dominated by Planktothrix agardhii (Oscillatoriales, Cyanophyta). Eur J Phycol 45:365–374
Kokociński M, Mankiewicz-Boczek J, Jurczak T, Spoof L, Meriluoto J, Rejmonczyk E, Hautala H, Vehniäinen M, Pawełczyk J, Soininen J (2013) Aphanizomenon gracile (Nostocales), a cylindrospermopsin-producing cyanobacterium in Polish lakes. Environ Sci Pollut Res Int 20:5243–5264
Kokocinski M, Gagala I, Jasser I, Karosiene J, Kasperoviciene J, Kobos J, Koreiviene J, Soininen J, Szczurowska A, Woszczyk M, Mankiewicz-Boczek J (2017) Distribution of invasive Cylindrospermopsis raciborskii in the East-Central Europe is driven by climatic and local environmental variables. FEMS Microbiol Ecol 93(4)
Komárek J (2013) Cyanoprokaryota. 3rd part: Heterocytous genera. In: Büdel B, Gärtner G, Krienitz L, Schagerl M (eds) Süßwasserflora von Mitteleuropa, 19/3. Springer Spektrum, Berlin Heidelberg, 1130 pp
Komárek J, Anagnostidis K (1998) Cyanoprokaryota 1. Teil: Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa 19/1, Gustav Fischer, Jena-Stuttgart-Lübeck-Ulm, 548 pp
Komárek J, Anagnostidis K (2005) Cyanoprokaryota 2. Teil/ 2nd Part: Oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (eds) Süsswasserflora von Mitteleuropa 19/2, Elsevier/Spektrum, Heidelberg, 759 pp
Lei L, Li C, Peng L, Han BP (2015) Competition between toxic and non-toxic Microcystis aeruginosa and its ecological implication. Ecotoxicology 24(7–8):1411–1418
Novoselova TN, Protasov AA (2016) Findings of cyanobacteria of tropical and subtropical origin in technoecosystems of NPP and TPP of Ukraine. Hydrobiol J 52:103–107
O’Neil JM, Davis TW, Burford MA, Gobler CJ (2012) The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae 14:313–334
Osswald J, Rellán S, Gago-Martinez A, Vasconcelos V (2009) Production of anatoxin-a by cyanobacterial strains isolated from Portuguese fresh water systems. Ecotoxicology 18:1110–1115
Paerl HW, Paul VJ (2011) Climate change: links to global expansion of harmful cyanobacteria. Water Res 46:1349–1363
Park BS, Li Z, Kang YH, Shin HH, Joo JH, Han MS (2018) Distinct bloom dynamics of toxic and non-toxic Microcystis (cyanobacteria) subpopulations in Hoedong Reservoir (Korea). Microb Ecol 75:163–173
Peryt TM, Durakiewicz T, Peryt D, Poberezhskyy A (2012) Carbon and oxygen isotopic composition of the Middle Miocene. Geol Acta 10:1–12
Rzymski P, Poniedziałek B (2014) In search of environmental role of cylindrospermopsin: a review on global distribution and ecology of its producers. Water Res 66:320–327
Rzymski P, Niedzielski P, Klimaszyk P, Poniedziałek B (2014a) Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ Monit Assess 186:3199–3212
Rzymski P, Poniedziałek B, Kokociński M, Jurczak T, Lipski D, Wiktorowicz K (2014b) Interspecific allelopathy in cyanobacteria: cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae 35:1–8
Rzymski P, Poniedziałek B, Mankiewicz-Boczek J, Faassen EJ, Jurczak T, Gągała-Borowska I, Ballot A, Lürling M, Kokociński M (2017a) Polyphasic toxicological screening of Cylindrospermopsis raciborskii and Aphanizomenon gracile isolated in Poland. Algal Res 24:72–80
Rzymski P, Brygider A, Kokociński (2017b) On the occurrence and toxicity of Cylindrospermopsis raciborskii in Poland. Limnol Rev 17:23–30
Rzymski P, Klimaszyk P, Marszelewski W, Borowiak D, Mleczek M, Nowiński K, Pius B, Niedzielski P, Poniedziałek B (2017c) The chemistry and toxicity of discharge waters from copper mine tailing impoundment in the valley of the Apuseni Mountains in Romania Environ Sci Pollut Res 24:21445–21458
Selheim F, Herfindal L, Martins R, Vasconcelos V, Doskeland SO (2005) Neuro-apoptogenic and blood platelet targeting toxins in benthic marine cyanobacteria from the Portuguese coast. Aquat Toxicol 74:294–306
Shcherbak VI, Bondarenko OV (2005) Spatial and temporal dynamics of phytoplankton in the “River-Reservoir-River” system. Hydrobiol J 41:36–41
Skuja H (1937) Süßwasseralgen aus Griechenland und Kleinasien. Hedwigia 77:15–73
Smutná M, Priebojová J, Večerková J, Hilscherová K (2016) Retinoid-like compounds produced by phytoplankton affect embryonic development of Xenopus laevis. Ecotoxicol Environ Saf 138:32–38
Suominen S, Brauer VS, Rantala-Ylinen A, Sivonen K, Hiltunen T (2017) Competition between a toxic and a non-toxic Microcystis strain under constant and pulsed nitrogen and phosphorus supply. Aquat Toxicol 51:117–130
Surface Water Sampling. SESDPROC-201-R3 (2013) Available on-line https://www.epa.gov/sites/production/files/2015-06/documents/Surfacewater-Sampling.pdf. Accessed 21.01.18
Wetzel RG, Likens GE (1991) Limnological analyses. Springer Verlag, New York, Berlin, Heidelberg, pp. 391
Yéprémian C, Gugger MF, Briand E, Catherine A, Berger C, Quiblier C, Bernard C (2007) Microcystin ecotypes in a perennial Planktothrix agardhii bloom. Water Res 41:4446–4456
Funding
This work has been partially supported by the Ministry of Education and Science of Ukraine (Project No. MV-1, 132B, 133B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vitor Manuel Oliveira Vasconcelos
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rzymski, P., Horyn, O., Budzyńska, A. et al. A report of Cylindrospermopsis raciborskii and other cyanobacteria in the water reservoirs of power plants in Ukraine. Environ Sci Pollut Res 25, 15245–15252 (2018). https://doi.org/10.1007/s11356-018-2010-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2010-6