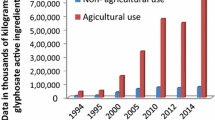
Abstract
Chemical analyses of the environment can document contamination by various xenobiotics, but it is also important to understand the effect of pollutants on living organisms. Thus, in the present work, we investigated the effect of the pesticide imidacloprid on the detoxifying enzyme glutathione S-transferase (GST) from Folsomia candida (Collembola), a standard test organism for estimating the effects of pesticides and environmental pollutants on non-target soil arthropods. Test animals were treated with different concentrations of imidacloprid for 48 h. Changes in steady-state levels of GST messenger RNA (mRNA) and GST enzyme activity were investigated. Extracted proteins were separated according to their sizes by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the resolved protein bands were detected by silver staining. The size of the glutathione (GSH) pool in Collembola was also determined. A predicted protein sequence of putative GSTs was identified with animals from control group. A 3-fold up-regulation of GST steady-state mRNA levels was detected in the samples treated with 5.0 mg L−1 imidacloprid compared to the control, while a 2.5- and 2.0- fold up-regulation was found in organisms treated with 2.5 and 7.5 mg L−1 imidacloprid, respectively. GST activity increased with increasing imidacloprid amounts from an initial activity of 0.11 μmol min−1 mg−1 protein in the control group up to 0.25 μmol min−1 mg−1 protein in the sample treated with the 5.0 mg L−1 of pesticide. By contrast, the total amount of GSH decreased with increasing imidacloprid concentration. The results suggest that the alteration of GST activity, steady-state level of GST mRNA, and GSH level may be involved in the response of F. candida to the exposure of imidacloprid and can be used as biomarkers to monitor the toxic effects of imidacloprid and other environmental pollutants on Collembola.




Similar content being viewed by others
References
Agianian B, Tucker PA, Schouten A, Leonard K, Bullard B, Gros P (2003) Structure of a Drosophila Sigma class glutathione S-transferase reveals a novel active site topography suited for lipid peroxidase products. J Mol Biol 326:151–165
Alves PRL, Cardoso EJBN, Martines AM, Sousa JP, Pasini A (2014) Seed dressing pesticides on springtails in two ecotoxicological laboratory tests. Ecotoxicol Environ Saf 105:65–71
Aly MAS, Schroeder P (2008) Effect of herbicides on glutathione S-transferases in the earthworm, Eisenia fetida. Environ Sci Pollut Res 15:143–149
Antunes SC, Marques SM, Pereira R, Goncalves F, Nunes B (2009) Testing procedures for the determination of several biomarkers in different species, for environmental assessment of pollution. J Environ Monit 12:1625–1630
Atkins WM, Wang RW, Bird AW, Newton DJ, Lu AHW (1993) The catalytic mechanism of glutathione S-transferase (GST): spectroscopic determination of the pKa of Tyr-9 in rat alpha 1-1 GST. J Biol Chem 268:19188–19191
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cal EPA (2006) Imidacloprid: risk characterization document-dietary and drinking water exposure
de Boer ME, de Boer TE, Marien J, Timmermans MJTN, Nota B, van Straalen NM, Ellers J, Roelofs D (2009) Reference genes for QRT-PCR tested under various stress conditions in Folsomia candida and Orchesella cincta (Insecta, Collembola). BMC Mol Biol 10(54):1–11
EASAC (2015) Ecosystem services, agriculture and neonicotinoids. EASAC policy report 26
EFSA (2008) Conclusion regarding the peer review of the pesticide risk assessment of the active substance imidacloprid
Enayati AA, Ranson H, Hemingway J (2005) Insect glutathione transferases and insecticide resistance. Insect Mol Biol 14:3–8
Fitzpatrick PJ, Sheehan D (1993) Separation of multiple forms of glutathione S-transferase from blue mussel, Mytilus edulis. Xenobiotica 23(8):851–861
Fitzpatrick PJ, Sheehan D, Livingstone DR (1995) Studies on isoenzymes of glutathione S-transferase in the digestive gland of Mytilus galloprovincialis with exposure to pollution. Mar Environ Res 39:241–244
Fountain MT, Hopkin SP (2001) Continuous monitoring of Folsomia candida (Insecta: Collembola) in a metal exposure test. Ecotoxicol Environ Saf 48:275–286
Fountain MT, Hopkin SP (2005) Folsomia candida (Collembola): a standard soil arthropod. Annu Rev Entomol 50:201–222
Fournier D, Bride JM, Poire M, Berge JB, Plapp FW (1992) Insect glutathione S-transferases: biochemical characteristics of the major forms from houseflies susceptible and resistant to insecticides. J Biol Chem 267:1840–1845
Gewande ND, Subashini SS, Muragan M, Subbarayalu M (2015) Molecular screening of insecticides with sigma glutathione S-transferases (GST) in cotton aphid Aphis gossypii using docking. Bioinformation 10(11):679–683
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Gruhlke MCH, Portz D, Stitz M, Anwar A, Schneider T, Jacob C, Schlaich NL, Slusarenko AJ (2010) Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Radical Biol Med 49:1916–1924
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hoffmann EJ (2008) Identification and characterization of key insecticide performance mechanisms for the control of plum curculio (Conotrachelus nenuphar) in Michigan tart cherries. Dissertation, Michigan State University
Johnson JA, Barbary AE, Kornguth SE, Brugge JF, Siegel FL (1993) Glutathione S-transferase isoenzymes in rat brain neurons and glia. J Neurosci 13(5):2013–2023
Klein O (1987) [14C]-NTN 33893: biokinetic part of the general metabolism study in rat. Report No. PF2889, dated 9 November 1987, submitted to WHO by Bayer AG, Mannheim, Germany. INCHEM toxicological evaluations: imidacloprid; International Programme on Chemical Safety. World Health Organization, Geneva
Kostaropoulos I, Papadopoulos AI (1998) Glutathione S-transferase isoenzymes expressed in the three developmental stages of insect Tenebrio molitor. Insect Biochem Mol 28:901–909
Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E (2001) Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem Mol 31:313–319
Lithner D, Nordensvan I, Dave G (2012) Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrite-butadiene-styrene, and epoxy to Daphnia magna. Environ Sci Pollut Res 19:1763–1772
Maria VL, Ribeiro MF, Amorim MJB (2014) Oxidative stress biomarkers and metallothionein in Folsomia candida—response to Cu and Cd. Environ Res 133:164–169
Marquini F, Picanco MC, Guedes RNC, Ferreira PSF (2003) Imidacloprid impact on arthropods associated with canopy of common bean. Neotrop Entomol 32(2):335–342
Nakamori T, Fujimori A, Kinoshita K, Ban-nai T, Kubota Y, Yoshida S (2010) mRNA expression of a cadmium-responsive gene is a sensitive biomarker of cadmium exposure in the soil collembolan Folsomia candida. Environ Pollut 158:1689–1695
NPIC (2015) Imidacloprid fact sheet. Oregon State University. http://npic.orst.edu/reports/NPIC14AR.pdf. Accessed 3 Feb 2015
OECD (2009) Guidelines for testing chemicals no. 232: Collembolan reproduction test in soil
Ribera D, Narbonne JF, Arnaud C, Saint-Denis M (2001) Biochemical responses of earthworm Eisenia fetida Andrei exposed to contaminated artificial soil, effects of carbaryl. Soil Biol Biochem 33:1123–1130
Saint-Denis M, Narbonne JF, Arnaud C, Thybaud E, Ribera D (1999) Biochemical responses of the earthworm Eisenia fetida Andrei exposed to contaminated artificial soil: effects of benzo(a)pyrene. Soil Biol Biochem 31:1837–1846
Shen S, Chien C (2003) Induction of glutathione S-transferases activities in Drosophila melanogaster exposed to phenol. Arch Insect Biochem 53:80–91
Stenersen J (2004) Chemical pesticides: mode of action and toxicity, 1st edn. CRC Press, Boca Raton
Stokke K, Stenersen J (1993) Non-inducibility of the glutathione transferases of the earthworm Eisenia ferida andrei. Comp Biochem Physiol 106C:753–756
Tomlin CDS (2000) A pesticide manual: a world compendium, 12th edn. BCPC publication, Berkshire
Wagner U, Edwards R, Dixon DP, Mauch F (2002) Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol Biol 49:515–532
Acknowledgments
This study was sponsored by the Ministry of Science and Technology, Thailand. We would like to thank Prof. Alan J. Slusarenko for advice and permission to use facilities in his laboratory and for critical reading of the manuscript. Thanks are also due to Dr. Martin C.H. Gruhlke and Dr. Marco Loehrer for their valuable discussions and technical help. We would like to express our gratefulness to Prof. Juliane Filser (University of Bremen, Germany) for kindly providing the F. candida culture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Sillapawattana, P., Schäffer, A. Effects of imidacloprid on detoxifying enzyme glutathione S-transferase on Folsomia candida (Collembola). Environ Sci Pollut Res 24, 11111–11119 (2017). https://doi.org/10.1007/s11356-016-6686-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6686-1