Abstract
Background
Inflammatory bowel disease is a group of pathologies characterised by chronic inflammation of the intestine and an unclear aetiology. Its main manifestations are Crohn’s disease and ulcerative colitis. Currently, biopsies are the most used diagnostic tests for these diseases and metabolomics could represent a less invasive approach to identify biomarkers of disease presence and progression.
Objectives
The lipid and the polar metabolite profile of plasma samples of patients affected by inflammatory bowel disease have been compared with healthy individuals with the aim to find their metabolomic differences. Also, a selected sub-set of samples was analysed following solid phase extraction to further characterise differences between pathological samples.
Methods
A total of 200 plasma samples were analysed using drift tube ion mobility coupled with time of flight mass spectrometry and liquid chromatography for the lipid metabolite profile analysis, while liquid chromatography coupled with triple quadrupole mass spectrometry was used for the polar metabolite profile analysis.
Results
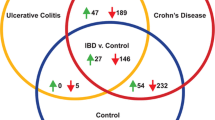
Variations in the lipid profile between inflammatory bowel disease and healthy individuals were highlighted. Phosphatidylcholines, lyso-phosphatidylcholines and fatty acids were significantly changed among pathological samples suggesting changes in phospholipase A2 and arachidonic acid metabolic pathways. Variations in the levels of cholesteryl esters and glycerophospholipids were also found. Furthermore, a decrease in amino acids levels suggests mucosal damage in inflammatory bowel disease.
Conclusions
Given good statistical results and predictive power of the model produced in our study, metabolomics can be considered as a valid tool to investigate inflammatory bowel disease.





Similar content being viewed by others
References
Agouridis, A. P., Elisaf, M., & Milionis, H. J. (2011). An overview of lipid abnormalities in patients with inflammatory bowel disease. Annals of Gastroenterology, 24(3), 181–187.
Ansell, B. J., Navab, M., Hama, S., Kamranpour, N., Fonarow, G., Hough, G., et al. (2003). Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation, 108(22), 2751–2756.
Balasubramanian, K., Kumar, S., Singh, R. R., Sharma, U., Ahuja, V., Makharia, G. K., et al. (2009). Metabolism of the colonic mucosa in patients with inflammatory bowel diseases: An in vitro proton magnetic resonance spectroscopy study. Magnetic Resonance Imaging, 27(1), 79–86.
Banks, C., Bateman, A., Payne, R., Johnson, P., & Sheron, N. (2003). Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. The Journal of Pathology, 199(1), 28–35.
Baumgart, D. C., & Carding, S. R. (2007). Inflammatory bowel disease: Cause and immunobiology. The Lancet, 369(9573), 1627–1640.
Berthold, M. R., & Hand, D. J. (2007). Intelligent data analysis: An introduction. Springer, Berlin.
Bischoff, S. C., Barbara, G., Buurman, W., Ockhuizen, T., Schulzke, J. D., Serino, M., et al. (2014). Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterology, 14(1), 189.
Biyyani, R. S. R. S., Putka, B. S., & Mullen, K. D. (2010). Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. Journal of Clinical Lipidology, 4(6), 478–482.
Blijlevens, N. M. A., Lutgens, L. C. H. W., Schattenberg, A. V. M. B., & Donnelly, J. P. (2004). Citrulline: A potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplantation, 34(3), 193.
Bruce, C., Chouinard, R. A. Jr., & Tall, A. R. (1998). Plasma lipid transfer proteins, high-density lipoproteins, and reverse cholesterol transport. Annual Review of Nutrition, 18(1), 297–330.
Cosnes, J., Gower–Rousseau, C., Seksik, P., & Cortot, A. (2011). Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology, 140(6), 1785–1794.
Dieterle, F., Ross, A., Schlotterbeck, G., & Senn, H. (2006). Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Analytical Chemistry, 78(13), 4281–4290.
Dunn, W. B., Broadhurst, D., Begley, P., Zelena, E., Francis-McIntyre, S., Anderson, N., et al. (2011). Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nature Protocols, 6(7), 1060.
Dunn, W. B., Wilson, I. D., Nicholls, A. W., & Broadhurst, D. (2012). The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis, 4(18), 2249–2264.
Ehehalt, R., Wagenblast, J., Erben, G., Lehmann, W. D., Hinz, U., Merle, U., et al. (2004). Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoelectrospray-tandem mass spectrometry. Scandinavian Journal of Gastroenterology, 39(8), 737–742.
Eriksson, L., Byrne, T., Johansson, E., Trygg, J., & Vikström, C. (2013). Multi-and megavariate data analysis basic principles and applications. Malmö: Umetrics Academy.
Fahy, E., Sud, M., Cotter, D., & Subramaniam, S. (2007). LIPID MAPS online tools for lipid research. Nucleic Acids Research, 35, W606–W612.
Fan, F., Mundra, P. A., Fang, L., Galvin, A., Moore, X. L., Weir, J. M., et al. (2015). Lipidomic profiling in inflammatory bowel disease: Comparison between ulcerative colitis and Crohn’s disease. Inflammatory Bowel Diseases, 21(7), 1511–1518.
Fraunberger, P., Nagel, D., Walli, A. K., & Seidel, D. (2000). Serum cholesterol and mortality in patients with multiple organ failure. Critical Care Medicine, 28, 3574–3575.
Han, X., & Gross, R. W. (2005). Shotgun lipidomics: Multidimensional MS analysis of cellular lipidomes. Expert Review of Proteomics, 2(2), 253–264.
Harvath, L., Robbins, J. D., Russell, A. A., & Seamon, K. B. (1991). cAMP and human neutrophil chemotaxis. Elevation of cAMP differentially affects chemotactic responsiveness. The Journal of Immunology, 146(1), 224–232.
Harvey, R. F., & Bradshaw, M. J. (1980). Measuring Crohn’s disease activity. Lancet, 1(8178), 1134–1135.
Hinz, C., Liggi, S., & Griffin, J. L. (2018). The potential of ion mobility mass spectrometry for high-throughput and high-resolution lipidomics. Current Opinion in Chemical Biology, 42, 42–50.
Hisamatsu, T., Okamoto, S., Hashimoto, M., Muramatsu, T., Andou, A., Uo, M., et al. (2012). Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PloS one, 7 (1), e31131.
Hong, S. K. S., Maltz, B. E., Coburn, L. A., Slaughter, J. C., Chaturvedi, R., Schwartz, D. A., et al. (2009). Increased serum levels of L-arginine in ulcerative colitis and correlation with disease severity. Inflammatory bowel diseases, 16(1), 105–111.
Kaser, A., Zeissig, S., & Blumberg, R. S. (2010). Inflammatory bowel disease. Annual Review of Immunology, 28, 573–621.
Kliman, M., May, J. C., & McLean, J. A. (2011). Lipid analysis and lipidomics by structurally selective ion mobility-mass spectrometry. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1811(11), 935–945.
Kominsky, D. J., Campbell, E. L., & Colgan, S. P. (2010). Metabolic shifts in immunity and inflammation. The Journal of Immunology, 184(8), 4062–4068.
Kumar, P., & Clark, M. L. (2016) Kumar and Clark’s clinical medicine (9th ed.). Amsterdam: Elsevier, pp. 309–319.
Kyle, J. E., Aly, N., Zheng, X., Burnum-Johnson, K. E., Smith, R. D., & Baker, E. S. (2018). Evaluating lipid mediator structural complexity using ion mobility spectrometry combined with mass spectrometry. Bioanalysis, 10(5), 279–289.
Liggi, S. (2018). sonial/KniMet: First release of KniMet (Version v1.2.0). Zenodo. https://doi.org/10.5281/zenodo.1196407.
Liggi, S., Hinz, C., Hall, Z., Santoru, M. L., Poddighe, S., Fjeldsted, J., et al. (2017). KniMet: A pipeline for the processing of chromatography–mass spectrometry metabolomics data. Metabolomics, 14(4), 52.
Lin, H. M., Helsby, N. A., Rowan, D. D., & Ferguson, L. R. (2011). Using metabolomic analysis to understand inflammatory bowel diseases. Inflammatory Bowel Diseases, 17(4), 1021–1029.
Lu, K., Knutson, C. G., Wishnok, J. S., Fox, J. G., & Tannenbaum, S. R. (2012). Serum metabolomics in a Helicobacter hepaticus mouse model of inflammatory bowel disease reveal important changes in the microbiome, serum peptides, and intermediary metabolism. Journal of Proteome Research, 11(10), 4916–4926.
Maul, J., Loddenkemper, C., Mundt, P., Berg, E., Giese, T., Stallmach, A., et al. (2005). Peripheral and intestinal regulatory CD4+ CD25 high T cells in inflammatory bowel disease. Gastroenterology, 128(7), 1868–1878.
Moore, A. R., & Willoughby, D. A. (1995). The role of cAMP regulation in controlling inflammation. Clinical & Experimental Immunology, 101(3), 387–389.
Niemelä, K., & Sjöström, E. (1986). Simultaneous identification of aromatic and aliphatic low molecular weight compounds from alkaline pulping liquor by capillary gas-liquid chromatography-mass spectrometry. Holzforschung-International Journal of the Biology, Chemistry, Physics and Technology of Wood, 40(6), 361–368.
Nikolaus, S., & Schreiber, S. (2007). Diagnostics of inflammatory bowel disease. Gastroenterology, 133(5), 1670–1689.
Paglia, G., Kliman, M., Claude, E., Geromanos, S., & Astarita, G. (2015). Applications of ion-mobility mass spectrometry for lipid analysis. Analytical and Bioanalytical Chemistry, 407(17), 4995–5007.
Peterson, J. W., Dickey, W. D., Saini, S. S., Gourley, W., Klimpel, G. R., & Chopra, A. K. (1996). Phospholipase A2 activating protein and idiopathic inflammatory bowel disease. Gut, 39(5), 698–704.
Rivkin, I., & Neutze, J. A. (1977). Influence of cyclic nucleotides and a phosphodiesterase inhibitor on in vitro human blood neutrophil chemotaxis. Archives internationales de pharmacodynamie et de therapie, 228(2), 196–204.
Ruxton, G. D. (2006). The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behavioral Ecology, 17(4), 688–690.
Santoru, M. L., Piras, C., Murgia, A., Palmas, V., Camboni, T., Liggi, S., et al. (2017). Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Scientific Reports, 7(1), 9523.
Schaloske, R. H., & Dennis, E. A. (2006). The phospholipase A2 superfamily and its group numbering system. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1761(11), 1246–1259.
Schmelzer, K., Fahy, E., Subramaniam, S., & Dennis, E. A. (2007). The lipid maps initiative in lipidomics. Methods in Enzymology, 432, 171–183.
Schroeder, K. W., Tremaine, W. J., & Ilstrup, D. M. (1987). Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. New England Journal of Medicine, 317(26), 1625–1629.
Scoville, E. A., Allaman, M. M., Brown, C. T., Motley, A. K., Horst, S. N., Williams, C. S., et al. (2018). Alterations in lipid, amino acid, and energy metabolism distinguish Crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics, 14(1), 17.
Steinbach, G., Morotomi, M., Nomoto, K., Lupton, J., Weinstein, I. B., & Holt, P. R. (1994). Calcium reduces the increased faecal 1, 2-sn-diacylglycerol content in intestinal bypass patients: A possible mechanism for altering colonic hyperproliferation. Cancer Research, 54(5), 1216–1219.
Storr, M., Vogel, H. J., & Schicho, R. (2013). Metabolomics: Is it useful for IBD? Current Opinion in Gastroenterology, 29(4), 378.
Stow, S. M., Causon, T. J., Zheng, X., Kurulugama, R. T., Mairinger, T., May, J. C., & Hann, S. (2017). An interlaboratory evaluation of drift tube ion mobility—mass spectrometry collision cross section measurements. Analytical Chemistry, 89(17), 9048–9055.
Sud, M., Fahy, E., Cotter, D., Brown, A., Dennis, E. A., Glass, C. K., et al. (2006). Lmsd: Lipid maps structure database. Nucleic Acids Research, 35(suppl_1), D527–D532.
Summers, R. W., Elliott, D. E., Qadir, K., Urban Jr, J. F., Thompson, R., & Weinstock, J. V. (2003). Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. The American Journal of Gastroenterology, 98(9), 2034–2041.
Vidal-Lletjós, S., Beaumont, M., Tomé, D., Benamouzig, R., Blachier, F., & Lan, A. (2017). Dietary protein and amino acid supplementation in inflammatory bowel disease course: What impact on the colonic mucosa? Nutrients, 9(3), 310.
Weinstock, J. V., Summers, R. W., Elliott, D. E., Qadir, K., Urban, J. F., & Thompson, R. (2002). The possible link between de-worming and the emergence of immunological disease. The Journal of Laboratory and Clinical Medicine, 139(6), 334–338.
Zhou, Z., Tu, J., Xiong, X., Shen, X., & Zhu, Z. J. (2017). LipidCCS: Prediction of collision cross-section values for lipids with high precision to support ion mobility–mass spectrometry-based lipidomics. Analytical Chemistry, 89(17), 9559–9566.
Acknowledgements
We thank John Fjeldsted and Christine Miller for their support in the Ion Mobility analyses. This study was funded by Agilent Technologies, Regione Autonoma della Sardegna (L.R.7/2007, Grant Number F71J12001180002), and the Medical Research Council UK (Grant Number MR/P011705/1).
Author information
Authors and Affiliations
Contributions
PC, LA, JLG, AM and PU conceived the study, directed the project and designed the experiments. AM, MLS, CP and SL, performed the lipid metabolite profile extraction of the plasma samples. AM and CM performed the polar metabolite profile extraction of plasma samples. AM, CH, JW, JD and SL performed metabolomics and lipidomics experiments and data analysis. AM, CH and ZH, contributed on the lipid targeted analysis. AM wrote the first draft of the manuscript, PC, LA, SL, CH, and JLG contributed to the final version. AM, SL, CH, JLG, PC and LA, critically reviewed the data and the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Comitato Etico Indipendente della A.O.U. di Cagliari via Ospedale, 54 - 09124 – Cagliari reference number: PG/2014/11480) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Murgia, A., Hinz, C., Liggi, S. et al. Italian cohort of patients affected by inflammatory bowel disease is characterised by variation in glycerophospholipid, free fatty acids and amino acid levels. Metabolomics 14, 140 (2018). https://doi.org/10.1007/s11306-018-1439-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1439-4