Abstract
Introduction
Biomarkers are needed in inflammatory bowel disease (IBD) to help define disease activity and identify underlying pathogenic mechanisms. We hypothesized that serum metabolomics, which produces unique metabolite profiles, can aid in this search.
Objectives
The aim of this study was to characterize serum metabolomic profiles in patients with IBD, and to assess for differences between patients with ulcerative colitis (UC), Crohn’s disease (CD), and non-IBD subjects.
Methods
Serum samples from 20 UC, 20 CD, and 20 non-IBD control subjects were obtained along with patient characteristics, including medication use and clinical disease activity. Non-targeted metabolomic profiling was performed using ultra-high performance liquid chromatography/mass spectrometry (UPLC-MS/MS) optimized for basic or acidic species and hydrophilic interaction liquid chromatography (HILIC/UPLC-MS/MS).
Results
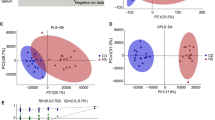
In total, 671 metabolites were identified. Comparing IBD and control subjects revealed 173 significantly altered metabolites (27 increased and 146 decreased). The majority of the alterations occurred in lipid-, amino acid-, and energy-related metabolites. Comparing only CD and control subjects revealed 286 significantly altered metabolites (54 increased and 232 decreased), whereas comparing UC and control subjects revealed only five significantly altered metabolites (all decreased). Hierarchal clustering using significant metabolites separated CD from UC and control subjects.
Conclusions
We demonstrate that a number of lipid-, amino acid-, and tricarboxylic acid cycle-related metabolites were significantly altered in IBD patients, more specifically in CD. Therefore, alterations in lipid and amino acid metabolism and energy homeostasis may play a key role in the pathogenesis of CD.





Similar content being viewed by others
References
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300.
Bjerrum, J. T., Steenholdt, C., Ainsworth, M., Nielsen, O. H., Reed, M. A., Atkins, K., et al. (2017). Metabonomics uncovers a reversible proatherogenic lipid profile during infliximab therapy of inflammatory bowel disease. BMC Medicine, 15(1), 184. https://doi.org/10.1186/s12916-017-0949-7.
Bjerrum, J. T., Wang, Y., Hao, F., Coskun, M., Ludwig, C., Gunther, U., et al. (2015). Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics, 11, 122–133. https://doi.org/10.1007/s11306-014-0677-3.
Coburn, L. A., Horst, S. N., Allaman, M. M., Brown, C. T., Williams, C. S., Hodges, M. E., et al. (2016). l-Arginine availability and metabolism is altered in ulcerative colitis. Inflammatory Bowel Diseases, 22(8), 1847–1858. https://doi.org/10.1097/mib.0000000000000790.
Coburn, L. A., Horst, S. N., Chaturvedi, R., Brown, C. T., Allaman, M. M., Scull, B. P., et al. (2013). High-throughput multi-analyte Luminex profiling implicates eotaxin-1 in ulcerative colitis. PLoS ONE, 8(12), e82300. https://doi.org/10.1371/journal.pone.0082300.
Dawiskiba, T., Deja, S., Mulak, A., Zabek, A., Jawien, E., Pawelka, D., et al. (2014). Serum and urine metabolomic fingerprinting in diagnostics of inflammatory bowel diseases. World Journal of Gastroenterol, 20(1), 163–174. https://doi.org/10.3748/wjg.v20.i1.163.
De Preter, V., Machiels, K., Joossens, M., Arijs, I., Matthys, C., Vermeire, S., et al. (2015). Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut, 64(3), 447–458. https://doi.org/10.1136/gutjnl-2013-306423.
Dehaven, C. D., Evans, A. M., Dai, H., & Lawton, K. A. (2010). Organization of GC/MS and LC/MS metabolomics data into chemical libraries. Journal of Cheminformatics, 2(1), 9. https://doi.org/10.1186/1758-2946-2-9.
Esteve-Comas, M., Ramirez, M., Fernandez-Banares, F., Abad-Lacruz, A., Gil, A., Cabre, E., et al. (1992). Plasma polyunsaturated fatty acid pattern in active inflammatory bowel disease. Gut, 33(10), 1365–1369.
Evans, A. M., DeHaven, C. D., Barrett, T., Mitchell, M., & Milgram, E. (2009). Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical Chemistry, 81(16), 6656–6667. https://doi.org/10.1021/ac901536h.
Fan, F., Mundra, P. A., Fang, L., Galvin, A., Moore, X. L., Weir, J. M., et al. (2015). Lipidomic profiling in inflammatory bowel disease: Comparison between ulcerative colitis and Crohn’s disease. Inflammatory Bowel Diseases, 21(7), 1511–1518. https://doi.org/10.1097/mib.0000000000000394.
Geboes, K., Colombel, J. F., Greenstein, A., Jewell, D. P., Sandborn, W. J., Vatn, M. H., et al. (2008). Indeterminate colitis: A review of the concept–what’s in a name? Inflammatory Bowel Diseases, 14(6), 850–857. https://doi.org/10.1002/ibd.20361.
Gnewuch, C., Liebisch, G., Langmann, T., Dieplinger, B., Mueller, T., Haltmayer, M., et al. (2009). Serum bile acid profiling reflects enterohepatic detoxification state and intestinal barrier function in inflammatory bowel disease. World Journal of Gastroenterol, 15(25), 3134–3141.
Goedert, J. J., Sampson, J. N., Moore, S. C., Xiao, Q., Xiong, X., Hayes, R. B., et al. (2014). Fecal metabolomics: Assay performance and association with colorectal cancer. Carcinogenesis, 35(9), 2089–2096. https://doi.org/10.1093/carcin/bgu131.
Gothe, F., Beigel, F., Rust, C., Hajji, M., Koletzko, S., & Freudenberg, F. (2014). Bile acid malabsorption assessed by 7 alpha-hydroxy-4-cholesten-3-one in pediatric inflammatory bowel disease: Correlation to clinical and laboratory findings. Journal of Crohn’s and Colitis, 8(9), 1072–1078.
Gregor, J. C., McDonald, J. W., Klar, N., Wall, R., Atkinson, K., Lamba, B., et al. (1997). An evaluation of utility measurement in Crohn’s disease. Inflammatory Bowel Diseases, 3(4), 265–276.
Harvey, R. F., & Bradshaw, J. M. (1980). A simple index of Crohn’s-disease activity. The Lancet, 1(8167), 514.
Hendriksen, C., Kreiner, S., & Binder, V. (1985). Long term prognosis in ulcerative colitis–based on results from a regional patient group from the county of Copenhagen. Gut, 26(2), 158–163.
Hisamatsu, T., Okamoto, S., Hashimoto, M., Muramatsu, T., Andou, A., Uo, M., et al. (2012). Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS ONE, 7(1), e31131. https://doi.org/10.1371/journal.pone.0031131.
Hong, S. K., Maltz, B. E., Coburn, L. A., Slaughter, J. C., Chaturvedi, R., Schwartz, D. A., et al. (2010). Increased serum levels of l-arginine in ulcerative colitis and correlation with disease severity. Inflammatory Bowel Diseases, 16(1), 105–111. https://doi.org/10.1002/ibd.21035.
Kolho, K. L., Pessia, A., Jaakkola, T., de Vos, W. M., & Velagapudi, V. (2017). Faecal and serum metabolomics in paediatric inflammatory bowel disease. Journal of Crohn’s and Colitis, 11(3), 321–334. https://doi.org/10.1093/ecco-jcc/jjw158.
Koutroumpakis, E., Ramos-Rivers, C., Regueiro, M., Hashash, J. G., Barrie, A., Swoger, J., et al. (2016). Association between long-term lipid profiles and disease severity in a large Cohort of patients with inflammatory bowel disease. Digestive Diseases and Sciences, 61(3), 865–871. https://doi.org/10.1007/s10620-015-3932-1.
Lenicek, M., Duricova, D., Komarek, V., Gabrysova, B., Lukas, M., Smerhovsky, Z., et al. (2011). Bile acid malabsorption in inflammatory bowel disease: Assessment by serum markers. Inflammatory Bowel Diseases, 17(6), 1322–1327.
Lennie, T. A., McCarthy, D. O., & Keesey, R. E. (1995). Body energy status and the metabolic response to acute inflammation. American Journal of Physiology, 269(5 Pt 2), R1024–R1031.
Lin, H. M., Helsby, N. A., Rowan, D. D., & Ferguson, L. R. (2011). Using metabolomic analysis to understand inflammatory bowel diseases. Inflammatory Bowel Diseases, 17(4), 1021–1029. https://doi.org/10.1002/ibd.21426.
Marchesi, J. R., Holmes, E., Khan, F., Kochhar, S., Scanlan, P., Shanahan, F., et al. (2007). Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. Journal of Proteome Research, 6(2), 546–551. https://doi.org/10.1021/pr060470d.
Marion-Letellier, R., Savoye, G., Beck, P. L., Panaccione, R., & Ghosh, S. (2013). Polyunsaturated fatty acids in inflammatory bowel diseases: A reappraisal of effects and therapeutic approaches. Inflammatory Bowel Diseases, 19(3), 650–661. https://doi.org/10.1097/MIB.0b013e3182810122.
Ooi, M., Nishiumi, S., Yoshie, T., Shiomi, Y., Kohashi, M., Fukunaga, K., et al. (2011). GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflammation Research, 60(9), 831–840. https://doi.org/10.1007/s00011-011-0340-7.
Playdon, M. C., Sampson, J. N., Cross, A. J., Sinha, R., Guertin, K. A., Moy, K. A., et al. (2016). Comparing metabolite profiles of habitual diet in serum and urine. The American Journal of Clinical Nutrition, 104(3), 776–789. https://doi.org/10.3945/ajcn.116.135301.
Podolsky, D. K. (2002). Inflammatory bowel disease. New England Journal of Medicine, 347(6), 417–429. https://doi.org/10.1056/NEJMra020831.
Ricart, E., Garcia-Bosch, O., Ordas, I., & Panes, J. (2008). Are we giving biologics too late? The case for early versus late use. World Journal of Gastroenterol, 14(36), 5523–5527.
Schicho, R., Shaykhutdinov, R., Ngo, J., Nazyrova, A., Schneider, C., Panaccione, R., et al. (2012). Quantitative metabolomic profiling of serum, plasma, and urine by 1H NMR spectroscopy discriminates between patients with inflammatory bowel disease and healthy individuals. Journal of Proteome Research, 11(6), 3344–3357. https://doi.org/10.1021/pr300139q.
Schoepfer, A. M., Dehlavi, M. A., Fournier, N., Safroneeva, E., Straumann, A., Pittet, V., et al. (2013). Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. The American Journal of Gastroenterology, 108(11), 1744–1753. https://doi.org/10.1038/ajg.2013.248. quiz 1754.
Schroeder, K. W., Tremaine, W. J., & Ilstrup, D. M. (1987). Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New England Journal of Medicine, 317(26), 1625–1629. https://doi.org/10.1056/nejm198712243172603.
Sharon, P., & Stenson, W. F. (1984). Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology, 86(3), 453–460.
Shivashankar, R., Tremaine, W. J., Harmsen, W. S., & Loftus, E. V. Jr. (2016). Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clinical Gastroenterology and Hepatology, 15(6), 857–863. https://doi.org/10.1016/j.cgh.2016.10.039.
Stephens, N. S., Siffledeen, J., Su, X., Murdoch, T. B., Fedorak, R. N., & Slupsky, C. M. (2013). Urinary NMR metabolomic profiles discriminate inflammatory bowel disease from healthy. Journal of Crohn’s and Colitis, 7(2), e42–e48. https://doi.org/10.1016/j.crohns.2012.04.019.
Ueda, Y., Kawakami, Y., Kunii, D., Okada, H., Azuma, M., Le, D. S., et al. (2008). Elevated concentrations of linoleic acid in erythrocyte membrane phospholipids in patients with inflammatory bowel disease. Nutrition Research, 28(4), 239–244. https://doi.org/10.1016/j.nutres.2008.02.005.
Vavricka, S. R., Spigaglia, S. M., Rogler, G., Pittet, V., Michetti, P., Felley, C., et al. (2012). Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflammatory Bowel Diseases, 18(3), 496–505. https://doi.org/10.1002/ibd.21719.
Vermeire, S., Van Assche, G., & Rutgeerts, P. (2006). Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut, 55(3), 426–431. https://doi.org/10.1136/gut.2005.069476.
Vítek, L. (2015). Bile acid malabsorption in inflammatory bowel disease. Inflammatory Bowel Diseases, 21(2), 476–483.
Wiese, D. M., Horst, S. N., Brown, C. T., Allaman, M. M., Hodges, M. E., Slaughter, J. C., et al. (2016). Serum fatty acids are correlated with inflammatory cytokines in ulcerative colitis. PLoS ONE, 11(5), e0156387. https://doi.org/10.1371/journal.pone.0156387.
Williams, H. R., Cox, I. J., Walker, D. G., North, B. V., Patel, V. M., Marshall, S. E., et al. (2009). Characterization of inflammatory bowel disease with urinary metabolic profiling. The American Journal of Gastroenterology, 104(6), 1435–1444. https://doi.org/10.1038/ajg.2009.175.
Williams, H. R., Willsmore, J. D., Cox, I. J., Walker, D. G., Cobbold, J. F., Taylor-Robinson, S. D., et al. (2012). Serum metabolic profiling in inflammatory bowel disease. Digestive Diseases and Sciences, 57(8), 2157–2165. https://doi.org/10.1007/s10620-012-2127-2.
Wu, G., & Morris, S. M. Jr. (1998). Arginine metabolism: Nitric oxide and beyond. Biochemical Journal, 336(Pt 1), 1–17.
Xavier, R. J., & Podolsky, D. K. (2007). Unravelling the pathogenesis of inflammatory bowel disease. Nature, 448(7152), 427–434. https://doi.org/10.1038/nature06005.
Zhang, Y., Lin, L., Xu, Y., Lin, Y., Jin, Y., & Zheng, C. (2013). 1H NMR-based spectroscopy detects metabolic alterations in serum of patients with early-stage ulcerative colitis. Biochemical and Biophysical Research Communications, 433(4), 547–551. https://doi.org/10.1016/j.bbrc.2013.03.012.
Zhou, G., Song, Y., Yang, W., Guo, Y., Fang, L., Chen, Y., et al. (2016). ASCA, ANCA, ALCA and many more: Are they useful in the diagnosis of inflammatory bowel disease? Digestive Diseases, 34(1–2), 90–97. https://doi.org/10.1159/000442934.
Funding
Supported by National Institutes of Health (NIH) Grants R01AT004821 and 3R01AT004821-02S1 to KTW. EAS was supported by NIH Training Grant 2T32HD060554-06A1. LAC was supported by NIH Training Grant 5T32DK007673, a Vanderbilt Physician Scientist Development Award, a Veterans Affairs Career Development Award 1IK2BX002126, and a Vanderbilt Digestive Disease Research Center Pilot and Feasibility Award from NIH Grant P30DK058404. Additional support was provided by NIH Grant P30DK058404 (Vanderbilt Digestive Disease Research Center), NIH Grant UL1TR000445 (Vanderbilt CTSA), the Vanderbilt Hormone Assay & Analytical Services Core supported by NIH Grant P30DK020593 (Vanderbilt Diabetes Research and Training Center), NIH R01DK099204 and Veterans Affairs Merit Review Grant I01BX001426 to CSW, NIH Grants R01DK053620, R01CA190612, P01CA028842, P01CA116087, and Veterans Affairs Merit Review Grant I01BX001453 to KTW, and the Thomas F. Frist Sr. Endowment to KTW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David A. Schwartz has consultancy agreements with Abbvie, UCB, Janssen, Takeda, and Tigenix. Dawn B. Beaulieu has a consultancy agreement with Abbvie. Sara N. Horst has consultancy agreements with UCB and Salix. However, these agreements and grants had no relationship to the current research study. Keith T. Wilson has had a consulting agreement with Immune Pharmaceuticals. However, this agreement had no relationship to the current research study and is no longer active. The remainder of the authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scoville, E.A., Allaman, M.M., Brown, C.T. et al. Alterations in lipid, amino acid, and energy metabolism distinguish Crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics 14, 17 (2018). https://doi.org/10.1007/s11306-017-1311-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1311-y