Abstract
Tropical forests are characterized by high biodiversity and aboveground biomass growing on strongly weathered soils. However, the distribution of plant species and soils are highly variable even within a tropical region. This paper reviews existing and novel knowledge on soil genesis, plant and microbial physiology, and biogeochemistry. Typically, forests in Southeast Asia are dominated by dipterocarps growing on acidic Ultisols from relatively young parent material. In the Neotropics and Africa, forests contain abundant legume trees growing on Oxisols developed in the older parent materials on stable continental shields. In Southeast Asia, the removal of base cations from the surface soil due to leaching and uptake by dipterocarp trees result in intensive acidification and accumulation of exchangeable Al3+, which is toxic to most plants. Nutrient mining by ectomycorrhizal fungi and efficient allocation within tree organs can supply phosphorus (P) for reproduction (e.g., mast fruiting) even on P-limited soils. In the Neotropics and Africa, nitrogen (N) fixation by legume trees can ameliorate N or P limitation but excess N can promote acidification through nitrification. Biological weathering [e.g., plant silicon (Si) cycling] and leaching can lead to loss of Si from soil. The resulting accumulation of Al and Fe oxides in Oxisols that can reduce P solubility through sorption and lead to limitation of P relative to N. Thus, geographical variation in geology and plant species drives patterns of soil weathering and niche differentiation at the global scale in tropical forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests share some similarities at the global scale (e.g., high productivity, rapid nutrient turnover, highly weathered soil, and low soil pH), but they also exhibit wide variation in soils and associated plant communities (Vitousek and Sanford 1986). In particular, different tropical regions (Southeast Asia, Africa, and Neotropics) possess distinct geological histories and plant communities (Corlett and Primack 2006). Variation in climate, geology, and topography can cause diverse patterns and processes of plants, soils, and their interactions (Vitousek 2004).
Several papers have reported correlations between soil properties, such as pH or P with forest properties, such as forest aboveground biomass or species distributions (Van Schaik and Mirmanto 1985; Terborgh 1992; John et al. 2007; Condit et al. 2013). In contrast, other studies have failed to link soil properties and plant species distribution due to the narrow variation of soil chemical properties and low resolution of soil classification (Sollins 1998). Comparison between tropical regions can provide variation in plant species (e.g., dipterocarp vs. legume trees), soil types (Ultisols vs. Oxisols), and availability of nutrients (N, P, and basic cations) with which to investigate roles of soils or plants on biodiversity and functions in tropical forests.
Soil is not a state factor for plant growth, as soil formation is influenced by plants (biota) as well as climate, geology, topography and time (Jenny 1994). Soil can affect plant physiological processes, while plants can also change soil processes (so-called plant-soil feedback). To answer the questions on cause-effect in plant-soil processes or plant-soil feedback requires the integration of knowledge on soil and plant science such as soil chronosequences, plant impacts on soil processes, strategies of nutrient acquisition and utilization (allocation), and niche differentiation related to soil nutrients.
In this review, we highlight the differences in geographical distribution of soil types (“Wide variation in tropical highly-weathered soils”) and vegetation and the other organisms (“Wide variation in vegetation and soil animals and microorganisms in tropical regions”) among tropical regions, and then discuss the effects of soil weathering on plant and microbial acquisition of nutrients (“Effects of pedogenesis on biological C, N, and P cycles in tropical forests”) and the effects of plant and microbial activities on soil formation (“Roles of plants and microorganisms on soil weathering in tropical forests”). Finally, we discuss plant-soil feedbacks in tropical forests (“Plant-soil feedbacks and edaphic niche differentiation in tropical forests”). It becomes possible to extract similarities and dissimilarities of the plant-soil interactions among tropical forests by comparing different tropical regions that hold distinct soil types and plants.
Wide variation in tropical highly-weathered soils
Diversity of tropical soils
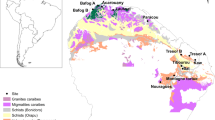
Tropical soils are regarded as highly weathered and infertile, but vary widely depending on climate, topography, and geology (Fig. 1). According to soil taxonomy (Soil Survey Staff 2006), the majority of tropical forest soils are classified primarily into five groups: Oxisols, Ultisols, Alfisols, Inceptisols, and Entisols (Sanchez and Logan 1992). Oxisols are distributed exclusively in tropical regions, whilst Ultisols are also distributed in warm temperate regions. Oxisols typically occur on stable landscapes of continental shields in Africa and South America. Oxisols correspond to Ferralsols in the World Reference Base for soil resources (WRB; FAO 2014) and Latosols in the Brazilian Soil Classification System. The so-called “laterite” refers to a narrow range of Oxisols that can form hardened horizons, or brick. The distribution patterns of Oxisols and Ultisols depend on geology and topography, as discussed later. The Ultisols and Alfisols share similarity in argillic (clay-illuviated) B horizons, but they differ in base saturation. The Ultisols have low base saturation (< 35%) and tend to be more acidic, whereas Alfisols typically form under drier conditions (tropical seasonal forest to savanna), have high base saturation and relatively high pH. Inceptisols and Entisols constitute significant proportions of tropical land (14 and 16%, respectively) independent of climate, and these pedogenetically young soils typically occur on hilly landscapes and floodplains (Sanchez and Logan 1992). Andisols constitute a minor proportion of the tropical land surface [1% from Sanchez and Logan (1992)]. They develop only on specific parent material of volcanic origin in Hawaii, Panama, Costa Rica, Puerto Rico, Sumatra, and Tanzania. Spodosols (or Podzols in WRB) are also rare in the tropics and are specific to vegetation (conifer), geology (sandy substrate), and landscapes (waterlogging). Histosols (or peat) are specific to flat landscapes affected by waterlogging and occur extensively in the tropics, but are particularly important in terms of their enormous stores of soil carbon (Page et al. 2011; Sjögersten et al. 2014).
Most tropical forest soils are similar in terms of their acidic pH and low base saturation. This is caused by intensive leaching over a long period of time under a climate where precipitation exceeds evapotranspiration (Fig. 2; Slessarev et al. 2016). This long-term weathering and leaching can cause soil acidification through depletion of carbonate and base cations (Eyre 1963).

(The data source is IGBP-DIS (1998))
Global distribution of acid soil. The shaded parts indicate acidic soils.
Comparison of physicochemical properties between Oxisols and Ultisols
Both Oxisols and Ultisols are regarded as highly-weathered soils, but extents and pathways of weathering are different (West et al. 1997; Do Nascimento et al. 2004, 2008). The differences in processes and products of weathering are recorded in clay mineralogy. Weathering of silicate clay minerals typically involves desilicification (loss of Si) from 2:1 type clay minerals (mica, vermiculite, smectite; an alumina sheet bounded by two silica sheets) and kaolinite (1:1 type clay minerals composed of silica sheet and alumina sheet) to the end products of gibbsite [Al(OH)3]. Oxisols are characterized by abundant kaolinite and Fe and Al oxides such as goethite (β-FeOOH), hematite (Fe2O3), and gibbsite (Schaefer et al. 2002). The Fe and Al oxides (and hydroxides) have pH-dependent charge and their clays have low cation exchange capacity (CEC), but they exhibit a large P sorption capacity (Uehara and Gillman 1981). Abundance of variable-charged oxides in Oxisols can contribute to the formation of stable micro-aggregate structure (2 to 250 μm in diameter) by binding oxides and kaolinite (Sollins et al. 1988). This typically allows good water drainage and development of deep root systems.
Compared to Oxisols, Ultisols typically have higher CEC derived from 2:1 type clay minerals such as vermiculite and smectite, with low base saturation (< 35%). The cation exchange sites are dominated by Al3+ ions. The concentrations of exchangeable Al3+ and K+ in the deeper horizons are greater in Ultisols than in Oxisols (Fig. 3). Ultisols are characterized by argillic horizons (clay illuviation) due to clay migration from surface horizon to deeper horizons (lessivage), but clay destruction by acidification can also contribute to loss of clays in the surface soil horizons (Fujii et al. 2009). The low concentrations of iron oxides and dominance of permanent-charged 2:1 type clay minerals lead to instability of soil structure and poor water drainage (Sollins et al. 1988).
Depth distribution of pH and exchangeable K, Ca, and Al in the soil profiles of Oxisol a and Ultisol b (unpublished data). Soil pH, exchangeable K and Ca, and exchangeable Al were measured by soil pH was measured in a 1:2 ratio of soil to deionized water, and exchangeable cations were determined by extraction in 0.1 M BaCl2, with detection by ICP optical emission spectrometry (Hendershot et al. 2008)
Geohistory and geology can explain the dominance of Ultisols in Southeast Asia and wide occurrence of Oxisols as well as Ultisols in Africa and South America. Oxisols commonly occur on continental shields in Africa and South America, where the Amazon and Congo plains share same old geological material from their association on the Gondwana supercontinent. In Southeast Asia, the distribution of Oxisols is limited to mafic and ultramafic parent materials (basalt and serpentine) (Fujii et al. 2011). Ultisols are commonly formed on Tertiary sedimentary rocks. The above-mentioned differences in soil physicochemical properties between Ultisols and Oxisols have a strong influence on patterns of plant distribution, microorganisms, and nutrient acquisition strategies between tropical regions.
Wide variation in vegetation and soil animals and microorganisms in tropical regions
Distribution of tropical trees
Tree communities vary markedly among tropical regions. The Dipterocarpaceae family dominates the forest canopy in Southeast Asia, whilst legume trees (e.g., Mimosoideae) are abundant in neotropical forests (Corlett and Primack 2011). The differences in dominant tree species are due to biogeographical and ecological reasons. Most of modern plant groups increased in diversity during the Tertiary (which began 66 million years ago) (Magallon et al. 1999). Angiosperm-dominated tropical forests increased in species diversity after the break-up of the Gonodwanan supercontinent in the middle Cretaceous (100 million years ago). The distance from center of the diversity and niche occupancy by the preceding vegetation groups can affect the present distribution of tree species (Corlett and Primack 2006).
The Dipterocarpaceae family originates from the Gondwana supercontinent, but travelled to Asia through drift of the Indian subcontinent (Ashton 1982). Phylogenic tree analyses suggests that Dipterocarpaceae diversified during the Miocene (23–5 million years ago) (Gunasekara 2004). Dipterocarpaceae exhibits large biomass production and high species diversity in Southeast Asia where Ultisols are dominant (Ashton et al. 1988). Dipterocarpaceae trees form associations with ectomycorrhizal (ECM) fungi and develop root mats on acidic soils, as typically seen in the temperate forests dominated by trees in Fagaceae trees, such as oaks and beeches (Ashton et al. 1988). In the Neotropics and Africa, most tree species associate with arbuscular mycorrhizal fungi, although forests dominated by ECM associated trees occur in both the Neotropics (e.g., Dicymbe forests in Guyana) and Africa (Gilbertiadendron forests in Central Africa) (McGuire 2007). The ECM fungi can release organic acids to solubilize minerals (function known as rock-eating fungi) and they can also produce enzymes to degrade organic matter and take up organic N (Chalot and Brun 1998; Landeweert et al. 2001), which can reduce N availability and promote monodominance (Corrales et al. 2016; see below). Some of ectomycorrhizal tree species tend to dominate forests and have reproductive patterns of mast-fruiting (Corlett and Primack 2006; McGuire 2007).
Legumes constitute a significant proportion of the canopy trees in tropical forests, especially in the Neotropics and Africa (Yahara et al. 2013). Since energetic costs to support N fixing organisms (N2-fixing bacteria known as rhizobia) are large, N fixation cannot always be an advantageous strategy compared to investment of C resources to root growth (Crews 1999). Consequence of competition between legume trees and non-fixers depend on various factors: light availability and the strength of N limitation, as well as other soil factors (P availability and low pH) (Crews 1999). Interestingly, legume trees account for a significant fraction of the tree community on N-rich soil in tropical lowland forests of Africa and the Neotropics (Houlton et al. 2008). Since phosphatase enzyme production requires N (containing up to 15% of N), phosphatase activity can be enhanced by increased N availability (Treseder and Vitousek 2001). Nitrogen fixation by legume trees and production of extracellular enzyme (phosphatases) by roots are therefore predicted to have advantages in acquiring P from organic matter in P-limited tropical soils (Houlton et al. 2008).
Distribution of soil animals and microorganisms in tropical forests
Soil animals and microorganisms are key drivers of biogeochemical cycles in plant-soil systems. In addition to differences in vegetation, the geographical distribution of the soil fauna also vary markedly among tropical regions. Termite (thought to be Gondwanan origin) are predominately soil-feeders in Africa, while in Southeast Asia, termite community are dominated by wood or litter feeders (Davies et al. 2003). Fungus-growing termites (Macrotermitinae with Termitomyces) rely on fungal cellulases for litter decomposition in Asia and Africa (Aanen et al. 2009), while leaf-cutting ants develop similar strategy (dependence on fungal cellulases) to decompose alive leaf in Neotropics (North et al. 1997). The stag beetles (Lucanidae), which form the earliest radiation of Scarabaeoidea, feed on dead woods and are commonly found in humid tropical forests, while the other groups of Scarabaeoidea that feed dung or humus are specialized to angiosperm species and their habitats (Ahrens et al. 2014).
In contrast to localization of soil fauna, most of microorganisms are considered to be cosmopolitan, because spores can be easily dispersed. There is a metaphor that “in microorganisms, everything is everywhere, the environment selects”, although this paradigm has not been supported by evidence (Foissner 2006). The environmental selection can cause differences in composition of soil microbial communities, but effects of their functions are not simple due to functional redundancy (Rousk et al. 2009). The microbial activity of soil organic matter decomposition is generally high under humid tropical climate (Hayakawa et al. 2013), leading to similarities among different tropical regions. On the other hand, variability in substrate (litter) quality and soil habitat causes wide variation in soil microbial communities and functions of nutrient cycles in forest soils.
Trees accumulate secondary metabolic compounds such as lignin and tannin for defense against herbivores, physical strength, and disease resistance (Kraus et al. 2003; Weng and Chapple 2010). The concentrations of lignin (measured by the Klason method) in leaf litters varies depending on tropical tree species [e.g., 11% (Bignoniaceae) to 45% (Dipterocarpaceae) from Ostertag et al. (2008) and Fujii et al. (2012)]. Lignin and tannin typically retard decomposition and N release in temperate forests, as lignin is generally more recalcitrant than cellulose due to complicated structure (Talbot and Treseder 2012). Based on the thermodynamic argument that enzymatic reactions metabolizing structurally complex, aromatic molecules such as lignin have higher activation energies and temperature dependence than reactions metabolizing structurally simpler molecules such as cellulose (Mikan et al. 2002), microbial lignin degradation in warmer climate, as well as litter fragmentation by soil animals (termite, ants, and earthworm etc.), can promote efficient litter decomposition and nutrient cycle in tropical forests (Fujii et al. 2012). The composition and functions of decomposer communities are influenced by climatic and local factors (e.g., lignin concentration in litters) (Talbot and Treseder 2012).
Regarding effects of soil environments on microbial communities, biomass or activities of fungi and bacteria can respond differently in response to changes in pH (Rousk et al. 2010). The relative contribution of fungi to total microbial respiration can increase with decreasing soil pH. Cellulose decomposition is limited in acidic soils (Hayakawa et al. 2013), while production of lignin-degrading peroxidases (lignin peroxidase and Mn peroxidase) by white-rot Basidiomycetes (Polyporales) is triggered under acidic and N-limited conditions of the Ultisols in dipterocarp forests of Southeast Asia (Fujii et al. 2012). On the other hand, fungal enzymes (laccase and Mn peroxidase) and some bacteria are involved in lignin degradation in Neotropical forests (Machado et al. 2005; DeAngelis et al. 2011).
Effects of pedogenesis on biological C, N, and P cycles in tropical forests
Changes in soil nutrient availability to plants and microorganisms
The pool sizes of soil nutrients are generally maintained by the balance between supply, transformation, and loss (Chapin et al. 2011). Intensive weathering and leaching in the humid tropics reduces soil nutrient concentrations and therefore nutrient availability to plants (Anderson 1988). Biogeochemical cycles differ among elements: the C and N can be fixed from CO2 and N2 gases in atmosphere, while basic cations can be supplied via precipitation as well as bedrock weathering (Vitousek 1984). On the other hand, the supply of P to the ecosystem is primarily dependent solely on bedrock, with relatively minor external inputs from aeolian dusts and volcanic ash, although such inputs can be an important source of P in some tropical regions, including the Amazon basin and Hawaii (e.g., Vitousek 2004). Therefore, highly weathered tropical soils tend to have less available P than the relatively young temperate soils due to a scarcity of easily-weatherable minerals and strong P fixation by Fe and Al oxides.
The initial P concentrations of geological substrates are variable; ultramafic rocks tend to contain low P concentrations, granite and sedimentary rocks are intermediate, and volcanic ash and lava tend to contain high P concentrations (Fujii et al. 2012, Porder and Ramachandran 2013). Soil P availability to plants can decrease with soil age (Walker and Syers 1976; Vitousek 1984; Turner et al. 2007). After the complete weathering of P in primary minerals (e.g., apatite), the dominant P forms are organic P and poorly soluble inorganic P (Turner and Engelbrecht 2011). Inorganic P is tightly bound to Fe and Al oxides, which are abundant in highly weathered soils such as Oxisols and Ultisols. As a result, productivity can be limited by poor soil P availability in tropical forests, which contrasts with N limitation in most the temperate forests (Vitousek 1984; Crews et al. 1995).
Nutrient demand for plant biomass production
It is essential to understand whether highly weathered soils can meet nutrient demands for production of biomass including litters, stems, and fruits. Phosphorus concentrations in aboveground biomass and litterfall are extremely low in Oxisols and Ultisols, but N concentrations are moderate (Vitousek and Sanford 1986; Townsend et al. 2007). Nitrogen input via litterfall is highly variable within tropical forests (28–224 kg N ha−1; Vitousek and Sanford 1986). The higher N inputs occur in forests dominated by legume trees, while the lower values occur in tropical montane forests on Spodosols.
Compared to N, P concentrations in litterfall are consistently low in tropical forests, although the P inputs via litterfall can vary from 1 to 15 kg P ha−1 due to the substantial amounts of litterfall input in tropical forests (Vitousek and Sanford 1986). The consistently low P concentrations in litterfall can be explained by the tight P cycle linked to low soil P availability and P resorption from fallen leaves (Hidaka and Kitayama 2009), as discussed later.
In addition to the annual nutrient demand for foliar litter production, supra-annual production of fruits at the community level is characteristic of dipterocarp forests in Southeast Asia (Janzen 1974). The triggers of massive flowering and consequent mass fruiting have been hypothesized to be related to climatic patterns [low temperature (Ashton et al. 1988) and/or droughts (Sakai et al. 2006) related to El Niño] and limitation of stored P resources in the tree related with low soil P availability (Ichie et al. 2005; Ichie and Nakagawa 2013). Dipterocarp fruits require more P (ca. 0.5 g P kg−1 dry matter) than litterfall (ca. 0.1 g P kg−1 dry matter). Based on the data of fruit production of 191 kg ha−1 from 48 tree species (Curran and Webb 2000), fruit production in the year requires substantial amounts of P (4.6 kg P ha−1 year−1). Based on the data of 10-year average P fluxes of fruit and flower production (0.7–1.4 kg P ha−1 year−1), greater percentages of P need to be allocated to reproductive organs (Kitayama et al. 2015). This nutrient demand reaches the same order of magnitude of annual N and P demand for annual litter production [3.0–3.7 kg P ha−1 year−1 from Fujii et al. (2011) and Kitayama et al. (2015)]. Phosphorus availability could therefore limit annual fruit production in dipterocarp forests in Southeast Asia.
Microbial responses to nutrient limitation in tropical soils
The biomass or activities of soil microorganisms are regulated by availability of nutrients as well as C resources (Schimel and Weintraub 2003). The nutrient demands of microorganisms fall within similar range (molar ratio of C:N = 7 (bacteria) and 5–17 (fungi), N:P = 7 (bacteria) and 15 (fungi) and extracellular enzymes (C:N = 3:1) (Cleveland and Liptzin 2007). This principle is applicable to a variety of tropical soils.
The high abundance of Al and Fe oxides in tropical soils (especially Oxisols) can increase P sorption and thus limit P availability to plants and microorganisms (Sollins et al. 1988). This contrasts with temperate coniferous forests, where slow decomposition of N-poor litter typically limits soil N availability (Reich et al. 1997). The high C:N ratios of organic substrates can lead to strong competition for N between microorganisms and plant roots (Schimel and Weintraub 2003). This holds true in some of tropical forests such as tropical montane forests invaded by conifers and tropical lowland forests dominated by non-legume trees (Kitayama et al. 2004; Fujii et al. 2009).
In N-limited ecosystems, some ECM fungi can decompose organic matter to obtain N by releasing extracellular enzymes (Chalot and Brun 1998). Ectomycorrhizal fungi are hypothesized to utilize soil organic N and increase the C:N ratio, which can decrease the decomposability of organic matter and increase soil C storage (Averill et al. 2014). This cycle can provide an advantage for growth of ECM fungi and associated trees against the saprotrophic fungi and bacteria and appear to promote monodominance by ECM trees in tropical forests (Corrales et al. 2016).
Regarding P deficiency, inorganic P can be released from organic P by phosphatase enzymes synthesized by plants and microorganisms. In addition, inorganic P bonded to oxides can be released through Fe solubilization by redox reactions caused by seasonal flooding (Peretyazhko and Sposito 2005) or by complexation with organic acids released from microorganisms and plant roots (Jones 1998). The microorganisms including ECM fungi have finer structures and wider surface area compared to plant roots. This is advantageous for plants to capture P, which is less mobile and more diffusion-dependent compared to N (Jones 1998).
Although competition for nutrients occurs between microorganisms and plants, soil microorganisms are not a permanent sink of nutrients. In tropical ecosystems, soil microbial biomass can fluctuate with wet-dry or seasonal cycles (Turner and Wright 2014), which can release N and P for plant uptake (Singh et al. 1989). The longer life span of trees than microorganisms can allow tree roots to take up nutrients when dead microorganisms supply substrates rich in N and P.
Roles of plants and microorganisms on soil weathering in tropical forests
Soil acidification by biological activities
In the humid tropics, the vast majority of soils are highly weathered and acidified because of intensive leaching over long periods of time (Eyre 1963; Slessarev et al. 2016). At the same time, soil acidification is a consequence of biogeochemical processes driven by plants and microorganisms such as nitrification, the dissociation of carbonic and organic acids, and excess cation uptake by plants (Van Breemen et al. 1983). Acidity generated by biological activity can promote mineral weathering and cation mobilization. Most tree species require greater amounts of cations (e.g., K+, Mg2+, Ca2+) than anions (e.g., Cl−, SO4 2−, H2PO4 −) and they release protons from roots to maintain their internal charge balance. The cation demands of plants in tropical forests are generally greater than in temperate forests due to the greater primary productivity in tropical forests (Fujii et al. 2010). Excess cation uptake by tropical plants therefore leads to high rates of soil acidification in tropical forests (Fujii 2014).
Different patterns of acidification can cause changes in soil chemical properties on pedogenic time scale (Ugolini and Sletten 1991). As acidification proceeds, different mechanisms are involved in acid neutralization, depending on pH ranges: buffering by carbonates (pH 6.2–8.6), weathering of primary minerals to secondary clay minerals (pH 5.0–6.2), cation exchange reactions (pH 4.2–5.0), and silicate clays (pH 3.8–4.2). Most tropical forest soils are in the pH ranges of cation exchange reactions and silicate clays. Al3+ is solubilized when protons replace exchangeable base cations and react with silicate clay structures. This Al3+ can occupy the cation exchangeable sites of 2:1 type silicate clays, if any, and increase exchangeable Al3+ in soil. Since much of the CEC is derived from 2:1 type silicate clays in Ultisols in Southeast Asia, acidification results in accumulation of the greater amounts of exchangeable Al3+, compared to Oxisols poor in silicate clays (Figs. 3, 4). Assuming that most of acidity can be neutralized within the profiles, cumulative impacts of acidification can be recorded as soil exchangeable Al3+. When cumulative proton generation for excessive uptake of cations over anions by plants (estimated from the charge balance between cations and anions in standing wood biomass) is compared with the size of soil exchangeable Al3+, soil exchangeable Al3+ are comparable to proton generation caused by cation uptake by plants (Fig. 5). The accordance between cumulative acidity in soil (exchangeable Al3+) and cumulative proton release by plants supports a high contribution of plants to soil acidification. On the other hand, the dominant process of acid neutralization in the Oxisols that are poor in 2:1 type silicate clays is different from Ultisols and, thus, exchangeable Al3+ of Oxisols is maintained at the lower level (Fig. 3).
Relationship between soil exchangeable Al3+ and acidity generated for excessive accumulation of cations over anions in standing wood biomass (a) and diagram to show the mechanism of accumulation of exchangeable Al3+ by plant cation uptake (b). Data sources include four sites of acidic soil (pH 4.0–5.3) and two sites of less acidic soil (pH 5.6–6.3) (Fujii et al. 2010, 2011). Soil depth to 30 cm was counted
Effects of plants on Si, Al, and Fe biogeochemistry
Aluminum, Fe, and Si constitute stable structural components of primary and secondary clay minerals, but they can be mobilized through weathering. Solubility of Al and Fe increases with decreasing pH. The solubility of Fe in the acidic condition is less than that of Al, but Fe solubility can increase under extremely acidic and/or reducing conditions. Silica (SiO2) solubility is generally greater under warm temperatures and at neutral pH (Krauskopf 1967; Verma 2000), while Si can be solubilized by acid weathering of silicate clay minerals in soils. The silicic acid (ortho-silicic acid H4SiO4) in soil solution can be supplied from both phytoliths (so-called plant opal) and silicate clays, but the ultimate source is the soil parent material (Lucas 2001). Therefore, the poverty of weatherable primary minerals can reduce Si availability to plants in tropical soils. Quartz (SiO2) is abundant even in highly-weathered tropical soils, but its solubility is lowest among the primary minerals (quartz ≪ olivine).
Mobilization of Al, Fe, and Si is not simply an abiotic process, but is also biological process mediated by plants and soil microorganisms. The chelating organic acids (e.g., citric acid) released by microorganisms (and/or reducing condition) contributes to the destruction of clays, leaching of Al and Fe from upper soil horizons, and their accumulation (illuviation) in the subsoil. This is the process that leads to the development of podzols (Spodosols in Soil Taxonomy) under temperate coniferous forests, but weak podzolization is also involved in the formation of Ultisols in tropical forests (Do Nascimento et al. 2004, 2008). Nutrient mining by ECM fungi associated with dipterocarp trees can promote the formation of an elluvial (E) horizon through organic acid exudation (Landeweert et al. 2001).
In contrast, carbonic acid produced by microbial and root respiration can contribute to incongruent dissolution of minerals. An absolute loss of Si (desilicification) results in a relative accumulation of Al and Fe oxides (ferralitization) in tropical soils, especially in Oxisols. Biological Si cycling via plants can influence the loss or accumulation of Si in soils. Rice, banana, and bamboo are well-known for Si accumulation in plant tissues, where Si can enhance physical strength (leaf architecture to receive sunlight) and defense against herbivores and pathogens (Street-Perrott and Barker 2008). The active uptake and accumulation of Si is driven by Si transporters in roots (Ma et al. 2006). High Si concentrations in litter were also observed for tropical woody species (Lucas 2001). Decomposition of Si-rich litters can increase the equilibrium Si concentrations in soil solution and increase the thermodynamic stability of kaolinite and halloysite even in gibbsite-dominated soil profiles, where otherwise the low equilibrium Si concentrations in soil solution can promote weathering of kaolinite or halloysite to gibbsite (Lucas et al. 1993; Lucas 2001; Kleber et al. 2007). On the other hand, plant Si cycling is estimated to drive Si loss and formation of Oxisol from basalt (Street-Perrott and Barker 2008).
Plant-soil feedbacks and edaphic niche differentiation in tropical forests
Plants and microorganisms can modify the soil environment and adjust to soil nutrient deficiency. The interactions between soil fertility and plant performance have been confirmed for Metrosideros polymorpha forests in the Hawaiian Island model system (Vitousek 2004). Low nutrient availability (especially P) in old P-limited soils leads to lower nutrient concentrations in plant tissues, longer lifespan of plant tissues and the nutrients within it, and greater resorption of nutrients to perennial organs (Hidaka and Kitayama 2009). This process can reduce litter nutrient concentrations and retard litter decomposition. This cycle accentuates low nutrient availability in soils (Vitousek 2004).
Soil acidification can also trigger plant-soil feedbacks (Fujii 2014; Fig. 6). In acidic soils on sandstone, low base cations and P concentrations in soils reduces the corresponding concentrations in plant tissues and increases nutrient use efficiency (Schlesinger et al. 1989). Low P concentrations in litters can increase the high C:P ratio of soil organic matter (Turner and Engelbrecht 2011) and the production of dissolved organic acids at low P availability due to limited microbial mineralization (Wieder et al. 2008; Fujii et al. 2011). Since dissolved organic acids are an important proton source in the surface soil (Ugolini and Sletten 1991), leaching of dissolved organic acids can further accentuate soil acidification (Fujii et al. 2009). The direction of feedback is influenced by the initial condition of the parent material, soil age, and leaching intensity. The wide variability of soil acidification patterns can originate from differences in climate, geology, and topography, but feedbacks between soil–plant-microorganisms cycle reinforce the direction and magnitude of soil acidification (Vitousek 2004).
An example of possible plant-soil feedback in the highly acidic Ultisol under dipterocarp forest. The bold arrows indicate process linkages, while the dot arrows indicate influence of state factors. The figure was drawn based on Fujii (2014)
When competition for nutrients exists between plant species in tropical forests with diverse plant community structure, edaphic niche partitioning or shifts in plant community structure can occur along with climate, water and light availability, and soil fertility (pH and nutrient availability) (Paoli et al. 2006). Shifts in plant community structure have been observed along soil acidity and fertility gradients of Panamanian tropical forests (John et al. 2007; Condit et al. 2013; Heineman et al. 2016). Some plants can survive on acidic soils by mitigating Al toxicity by complexation of Al3+ with organic acids released from roots (Fujii 2014; Heineman et al. 2016). The shifts in plant community structure toward efficient P utilizers can maintain high biomass production even in P-limited environments (Kitayama et al. 2000, 2004). Production of root phosphatases can be stimulated by P deficiency (Kitayama 2013; Ushio et al. 2015). In montane tropical forests of Malaysia and New Zealand, for example, soil aging (podzolization) is often associated with invasion of conifers (Podocarpaceae) (Kitayama et al. 2004). Tree species with greater organic acid excretion from roots can survive on podzolized soil of low P availability by acquiring P from the rhizosphere soil (Aoki et al. 2012). Differences in capacities to absorb N from N-limited soil between seedlings of some conifers (e.g. Dacrydium) and broadleaved trees can successfully explain shift in plant community structure with soil aging (Ushio et al. 2017). Identifying the main driving mechanisms of plant soil interactions would contribute to better understanding or prediction of forest dynamics and soil formation.
Conclusion
Variations in soil chemical properties (pH, exchangeable Al3+, Fe oxides, and P concentration) and nutrient cycling are large within tropical soils. These variations can be primarily explained by climate (e.g., leaching intensity and duration of droughts, including El Niño) and geology (geological age, silicate clays, Fe oxides, and bedrock P concentration). This paper emphasized the effects of plants on soil formation and the effects of soil properties on plants (ECM association, N2 fixation, Si accumulation by plants). These factors are not exclusive, but geographical variation in climate, geology, and plant species can be the triggers and drivers of plant-soil feedbacks, which drive diverse patterns of soil weathering on pedogenetic time scales. Comparison of tropical regions is promising to unravel the hidden roles of soils on biodiversity and functions in tropical forests.
References
Aanen DK, Henrik H, Debets AJ, Kerstes NA, Hoekstra RF, Boomsma JJ (2009) High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science 326:1103–1106
Ahrens D, Schwarzer J, Vogler AP (2014) The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proc R Soc Lond B 281:20141470
Andersen KM, Turner BL, Dalling JW (2009) Soil-based habitat partitioning in understorey palms in lower montane tropical forests. J Biogeogr 37:278–292
Anderson DW (1988) The effect of parent material and soil development on nutrient cycling in temperate ecosystems. Biogeochemistry 5:71–97
Aoki M, Fujii K, Kitayama K (2012) Environmental control of root exudation of low-molecular-weight organic acids in tropical rainforests. Ecosystems 15:1194–1203
Ashton PS (1982) Dipterocarpaceae. Flora Malesiana, series 1, vol 9. Martinus Nijhoff Publishers, The Hague
Ashton PS, Givnish TJ, Appanah S (1988) Staggered flowering in three Dipterocarpaceae: new insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. Am Nat 132:44–66
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545
Chalot M, Brun A (1998) Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas. FEMS Microbiol Rev 22:21–44
Chapin FS III, Matson PA, Vitousek P (2011) Principles of terrestrial ecosystem ecology. Springer Science & Business Media, Berlin
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Condit R, Engelbrecht BMJ, Pino D, Pérez R, Turner BL (2013) Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc Nat Acad Sci 110:5064–5068
Corlett RT, Primack RB (2006) Tropical rainforests and the need for cross-continental comparisons. Trends Ecol Evol 21:104–110
Corlett RT, Primack RB (2011) Tropical rain forests: an ecological and biogeographical comparison. Wiley, Hoboken
Corrales A, Mangan SA, Turner BL, Dalling JW (2016) An ectomycorrhizal nitrogen economy facilitates monodominance in a neotropical forest. Ecol Lett 19:383–392
Crews TE (1999) The presence of nitrogen fixing legumes in terrestrial communities: evolutionary vs ecological considerations. Biogeochemistry 46:233–246
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–1424
Curran LM, Webb CO (2000) Experimental tests of the spatiotemporal scale of seed predation in mast-fruiting Dipterocarpaceae. Ecol Monogr 70:129–148
Davies RG, Eggleton P, Jones DT, Gathorne-Hardy FJ, Hernández LM (2003) Evolution of termite functional diversity: analysis and synthesis of local ecological and regional influences on local species richness. J Biogeogr 30:847–877
DeAngelis KM, Allgaier M, Chavarria Y, Fortney JL, Hugenholtz P, Simmons B, Sublette K, Silver WL, Hazen TC (2011) Characterization of trapped lignin-degrading microbes in tropical forest soil. PLoS ONE 6:e19306
Do Nascimento NR, Bueno GT, Fritsch E, Herbillon AJ, Allard Th, Melfi AJ, Astolfo R, Boucher H, Li Y (2004) Podzolization as a deferralitization process: a study of an Acrisol-Podzol sequence derived from Palaeozoic sandstones in the northern upper Amazon Basin. Eur J Soil Sci 55:523–538
Do Nascimento NR, Fritsch E, Bueno GT, Bardy M, Grimaldi C, Melfi AJ (2008) Podzolization as a deferralitization process: dynamics and chemistry of ground and surface waters in an Acrisol-Podzol sequence of the upper Amazon Basin. Eur J Soil Sci 59:911–924
Eyre SR (1963) Vegetation and soils: a world picture. Edward Arnold, London
Foissner W (2006) Biogeography and dispersal of micro-organisms: a review emphasizing protists. Act Protozool 45:111–136
Fujii K (2014) Soil acidification and adaptations of plants and microorganisms in Bornean tropical forests. Ecol Res 29:371–381
Fujii K, Uemura M, Funakawa S, Hayakawa C, Sukartiningsih Kosaki T, Ohta S (2009) Fluxes of dissolved organic carbon in two tropical forest ecosystems of East Kalimantan, Indonesia. Geoderma 152:127–136
Fujii K, Hartono A, Funakawa S, Uemura M, Sukartiningsih Kosaki T (2010) Acidification of tropical forest soils derived from serpentine and sedimentary rocks in East Kalimantan, Indonesia. Geoderma 160:311–323
Fujii K, Hartono A, Funakawa S, Uemura M, Sukartiningsih Kosaki T (2011) Distribution of Ultisols and Oxisols in the serpentine areas of East Kalimantan, Indonesia. Pedologist 55:63–76
Fujii K, Uemura M, Hayakawa C, Funakawa S, Kosaki T (2012) Environmental control of lignin peroxidase, manganese peroxidase, and laccase activities in forest floor layers in humid Asia. Soil Biol Biochem 57:109–115
Gunasekara N (2004) Phylogenetic and molecular dating analyses of the tropical tree family Dipterocarpaceae based on chloroplast matK nucleotide sequence data. Doctoral dissertation, Concordia University
Hayakawa C, Funakawa S, Fujii K, Kadono A, Kosaki T (2013) Effects of climatic and soil properties on cellulose decomposition rates in temperate and tropical forests. Biol Fertil Soil 50:633–643
Heineman KD, Turner BL, Dalling JW (2016) Variation in wood nutrients along a tropical soil fertility gradient. New Phytol 211:440–454
Hendershot WH, Lalande H, Duquette M (2008) Ion exchange and exchangeable cations. In: Carter MR, Gregorich E (eds) Soil sampling and methods of analysis, chap 18. Canadian Society of Soil Science and CRC Press, Boca Raton, FL, pp 173–178
Hidaka A, Kitayama K (2009) Divergent patterns of photosynthetic phosphorus-use efficiency versus nitrogen-use efficiency of tree leaves along nutrient-availability gradients. J Ecol 97:984–991
Houlton BZ, Wang YP, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330
Ichie T, Nakagawa M (2013) Dynamics of mineral nutrient storage for mast reproduction in the tropical emergent tree Dryobalanops aromatica. Ecol Res 28:151–158
Ichie T, Tanaka K, Nakagawa M, Sato K, Nakashizuka T (2005) Resource allocation to reproductive organs during masting in the tropical emergent tree, Dipterocarpus tempehes. J Trop Ecol 21:237–241
IGBP-DIS (1998) Soil (V.0) data aprogram for creating global soil property databases. IGBP Global Soils Data Task, France
Janzen DH (1974) Tropical blackwater rivers, animals, and mast fruiting by the Dipterocarpaceae. Biotropica 6:69–103
Jenny H (1994) Factors of soil formation: a system of quantitative pedology. Courier Corporation, New York
John R, Dalling JW, Harms KE, Yavitt JB, Stallard RF, Mirabello M, Hubbell SP, Valencia R, Navarrete H, Vallejo M, Foster RB (2007) Soil nutrients influence spatial distributions of tropical tree species. Proc Nat Acad Sci 104:864–869
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Kitayama K (2013) The activities of soil and root acid phosphatase in the nine tropical rain forests that differ in phosphorus availability on Mount Kinabalu, Borneo. Plant Soil 37:215–224
Kitayama K, Majalap-Lee N, Aiba S (2000) Soil phosphorus fractionation and phosphorus-use efficiencies of tropical rainforests along altitudinal gradients of Mount Kinabalu, Borneo. Oecologia 123:342–349
Kitayama K, Aiba S, Takyu M, Majalap N, Wagai R (2004) Soil phosphorus fractionation and phosphorus-use efficiency of a Bornean tropical montane rain forest during soil aging with podozolization. Ecosystems 7:259–274
Kitayama K, Tsujii Y, Aoyagi R, Aiba S (2015) Long-term C, N and P allocation to reproduction in Bornean tropical rain forests. J Ecol 103:606–615
Kleber M, Schwendenmann L, Veldkamp E, Rößner J, Jahn R (2007) Halloysite versus gibbsite: silicon cycling as a pedogenetic process in two lowland neotropical rain forests soils of La Selva, Costa Rica. Geoderma 138:1–11
Kraus TE, Dahlgren RA, Zasoski RJ (2003) Tannins in nutrient dynamics of forest ecosystems-a review. Plant Soil 256:41–66
Krauskopf KB (1967) Introduction to geochemistry. McGraw-Hill, New York, p 721
Landeweert R, Hoffland E, Finlay RD, Kuyper TW, van Breemen N (2001) Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol Evol 16:248–254
Lucas Y (2001) The role of plants in controlling rates and products of weathering: importance of biological pumping. Ann Rev Earth Planet Sci 29:135–163
Lucas Y, Luizão FJ, Chauvel A, Rouiller J, Nahon D (1993) The relation between biological activity of the rain forest and mineral composition of soils. Science 260:521–523
Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Machado KM, Matheus DR, Bononi VL (2005) Ligninolytic enzymes production and remazol brilliant blue R decolorization by tropical Brazilian basidiomycetes fungi. Brazil J Microbiol 36:246–252
Magallon S, Crane PR, Herendeen PS (1999) Phylogenetic pattern, diversity, and diversification of eudicots. Ann Mo Bot Garden 86(2):297–372
McGuire KL (2007) Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88:567–574
Mikan CJ, Schimel JP, Doyle AP (2002) Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol Biochem 34:1785–1795
North RD, Jackson CW, Howse PE (1997) Evolutionary aspects of ant-fungus interactions in leaf-cutting ants. Trends Ecol Evol 12:386–389
Ostertag R, Marín-Spiotta E, Silver WL, Schulten J (2008) Litterfall and decomposition in relation to soil carbon pools along a secondary forest chronosequence in Puerto Rico. Ecosystems 11:701–714
Page SE, Rieley JO, Banks CJ (2011) Global and regional importance of the tropical peatland carbon pool. Glob Change Biol 17:798–818
Paoli GD, Curran LM, Zak DR (2006) Soil nutrients and beta diversity in the Bornean Dipterocarpaceae: evidence for niche partitioning by tropical rain forest trees. J Ecol 94:157–170
Peretyazhko T, Sposito G (2005) Iron (III) reduction and phosphorous solubilization in humid tropical forest soils. Geochim Cosmochim Acta 69:3643–3652
Porder S, Ramachandran S (2013) The phosphorus concentration of common rocks—a potential driver of ecosystem P status. Plant Soil 367:41–55
Reich PB, Grigal DF, Aber JD, Gower ST (1997) Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 78:335–347
Rousk J, Brookes PC, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 75:1589–1596
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME 4:1340–1351
Sakai S, Harrison RD, Momose K, Kuraji K, Nagamasu H, Yusunari T, Chong L, Nakashizuka T (2006) Irregular droughts trigger mass flowering in aseasonal tropical forests in Asia. Am J Bot 93:1134–1139
Sanchez PA, Logan TJ (1992) Myths and science about the chemistry and fertility of soils in the tropics. In: Lal R, Sanchez PA (eds) Myths and science of soils in the tropics, SSSA Special Publication 29. ASA and SSSA, Madison, pp 35–46
Schaefer CER, Ker JC, Gilkes RJ, Campos JC, de Costa LM, Saadi A (2002) Pedogenesis on the uplands of the Diamantina Plateau, Minas Gerais, Brazil: a chemical and micropedological study. Geoderma 107:243–269
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schlesinger WH, DeLucia EH, Billings WD (1989) Nutrient use efficiency of woody plants on contrasting soils in the Western Great Basin, Nevada. Ecology 70:105–113
Singh JS, Raghubanshi AS, Singh RS, Srivastava SC (1989) Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 338:499–500
Sjögersten S, Black CR, Evers S, Hoyos-Santillan J, Wright EL, Turner BL (2014) Tropical wetlands: a missing link in the global carbon cycle? Glob Biogeochem Cycles 28(12):1371–1386
Slessarev EW, Lin Y, Bingham NL, Johnson JE, Dai Y, Schimel JP, Chadwick OA (2016) Water balance creates a threshold in soil pH at the global scale. Nature 540:567–569
Soil Survey Staff (2006) Keys to soil taxonomy, 10th edn. United States Department of Agriculture Natural Resources Conservation Service, Washington, D.C.
Sollins P (1998) Factors influencing species composition in tropical lowland rain forest: does soil matter? Ecology 79:23–30
Sollins P, Robertson GP, Uehara G (1988) Nutrient mobility in variable-and permanent-charge soils. Biogeochemistry 6:181–199
Street-Perrott FA, Barker PA (2008) Biogenic silica: a neglected component of the coupled global continental biogeochemical cycles of carbon and silicon. Earth Surf Process Landf 33:1436–1457
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93:345–354
Terborgh J (1992) Diversity and the tropical rain forest. Freeman, New York, pp 31–104
Townsend AR, Cleveland CC, Asner GP, Bustamante M (2007) Controls over foliar N: P ratios in tropical rain forests. Ecology 88:107–118
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946–954
Turner BL, Engelbrecht BM (2011) Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103:297–315
Turner BL, Condron LM (2013) Pedogenesis, nutrient dynamics, and ecosystem development: the legacy of T.W. Walker and J.K. Syers. Plant Soil 367:1–10
Turner BL, Wright SJ (2014) The response of microbial biomass and hydrolytic enzyme activities to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 117:115–130
Turner BL, Condron LM, Richardson SJ, Peltzer DA, Allison VJ (2007) Soil organic phosphorus transformations during pedogenesis. Ecosystems 10:1166–1181
Uehara G, Gillman G (1981) The mineralogy, chemistry, and physics of tropical soils with variable charge clays. Westview Press, Boulder
Ugolini FC, Sletten RS (1991) The role of proton donors in pedogenesis as revealed by soil solution studies. Soil Sci 151:59–75
Ushio M, Fujiki Y, Hidaka A, Kitayama K (2015) Linkage of root physiology and morphology as an adaptation to soil phosphorus impoverishment in tropical montane forests. Funct Ecol 29:1235–1245
Ushio M, Aiba S-I, Takeuchi Y, Iida Y, Matsuoka S, Repin R, Kitayama K (2017) Plant-soil feedbacks and the dominance of conifers in a tropical montane forest in Borneo. Ecol Monogr 87:105–129
van Breemen N, Mulder J, Driscoll CT (1983) Acidification and alkalization of soils. Plant Soil 75:283–308
van Schaik CP, Mirmanto E (1985) Spatial variation in the structure and litterfall of a Sumatran rain forest. Biotropica 17:196–205
Verma MP (2000) Revised quartz solubility temperature dependence equation along the water–vapor saturation curve. In: Proceedings of the 2000 World Geothermal Congress. Kyushu, Tohoku, Japan, pp 1927–1932
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Vitousek PM (2004) Nutrient cycling and limitation: Hawai’i as a model system. Princeton University Press, Princeton
Vitousek PM, Sanford RL (1986) Nutrient cycling in moist tropical forest. Ann Rev Ecol Syst 17(1):137–167
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Weng JK, Chapple C (2010) The origin and evolution of lignin biosynthesis. New Phytol 187:273–285
West LT, Beinroth FH, Sumner ME, Kang BT (1997) Ultisols: characteristics and impacts on society. Adv Agron 63:179–236
Wieder WR, Cleveland CC, Townsend AR (2008) Tropical tree species composition affects the oxidation of dissolved organic matter from litter. Biogeochemistry 88:127–138
Yahara T et al (2013) Global legume diversity assessment: concepts, key indicators, and strategies. Taxon 62:249–266
Acknowledgements
This review paper was based on talks of Ecological Research Symposium of the 63th Annual Meeting of the Ecological Society of Japan held in 2016 Sendai. The travel fund was supported from Ecological Research and Ecological Society of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Fujii, K., Shibata, M., Kitajima, K. et al. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol Res 33, 149–160 (2018). https://doi.org/10.1007/s11284-017-1511-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-017-1511-y