Abstract
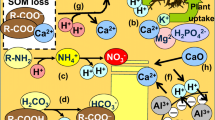
Acidification or alkalinization of soils occurs through H+ transfer processes involving vegetation, soil solution and soil minerals. A permanent change in the acid neutralizing capacity of the inorganic soil fraction (ANC(s)),i.e. soil acidification (ΔANC<0) or soil alkalinization (ΔANC>0), results from an irreversible H+ flux. This irreversible H+ flux can be caused either by direct proton addition or depletion, by different mobility of components of the ANC(s) or by a permanent change in redox conditions. The contributions of (a) acidic atmospheric deposition, (b) nitrogen transformations, (c) deprotonation of CO2 and of organic acids and protonation of their conjugate bases, (d) assimilation of cations and anions by the vegetation, (e) weathering or reverse weathering of minerals and (f) stream output to changes in the ANC(s) are illustrated by means of H+ budgets for actual soils and watersheds.
Similar content being viewed by others
References
Brinkman R 1970 Ferrolysis; a hydromorphic soil forming process. Geoderma 3, 199–206.
Bruggenwert M G M 1972 Adsorptie van Al-ionen aan het kleimineraal montmorilloniet. Versl. Landb. Onderz. 768. Pudoc, Wageningen, 120 p.
Bruynzeel L A 1983 Hydrological and biogeochemical aspects of man-made forests in south-central Java, Indonesia. Ph. D.-thesis Free Univ. Amsterdam.
Carmoure J P 1976 La régulation hydrogéochimique du lac Tchad. Trav. et Docum. de L. ORSTROM No. 58. ORSTOM, Paris.
Coleman N T and Thomas G W 1967 The basic chemistry of soil acidity.In Soil acidity and liming. The American Society of Agronomy. Madison. Wisconsin: 1–41.
De Wit C T, Dijkshoorn W and Noggle J C 1963 Ionic balance and growth of plants. Versl. Landbouwk. Onderz. 69. 15. Wageningen.
Driscoll C T 1980 Chemical characterization of some dilute acidified lakes and streams in the Adirondack region of New York State. Ph. D. Thesis, Dept. of Environmental Engineering, Cornell Univ.
Driscoll C T and Likens G E 1982 Hydrogen ion budget of an aggrading forested ecosystem. Tellus 34, 283–292.
Garrels R M and Mackenzie F T 1971 Evolution of Sedimentary Rocks. Norton, New York.
Hemond H F 1980. Biogeochemistry of Thoreau's Bog, Concord, Massachusetts. Ecological Monographs 50, 507–526.
Hoagland D R and Broyer T C 1936 General nature of the process of salt accumulation by roots, description of experimental methods. Plant Physiol. 11, 472–507.
Holland H D 1978 The Chemistry of the Atmosphere and Oceans. John Wiley. New York
Janitzky P and Whittig L D 1964 Mechanisms of formation of Na2CO3 in soils. II. Laboratory study of biogenesis. Soil Sci. 15, 145–157.
Likens G E, Bormann F H, Pierce R S, Eaton J S and Johnson N M 1977 Biogeochemistry of a forested ecosystem. Springer Verlag, New York, 146 p.
Likens G E, Bormann F H, Johnson N M, Fisher D W and Pierce R S 1970 Effects of forest cutting and herbicide treatment on nutrient budgets in the Hubbard Brook watershed-ecosystem. Ecol. Monographs 40, 23–47.
Lovelock J E 1979 Gaia. A new Look at Life on Earth. Oxford Univ. Press. Oxford.
Mattson S 1938 The constitution of the pedosphere. Landbrukshögskolans Ann. 5, 261–276.
Mattson S and Koutler-Andersson E 1941 The acid-base condition in vegetation, litter and humus: I. Acids, acidoids and bases in relation to decomposition. Landbrukshögskolans Ann. 9, 1–26.
Matzner E, Khanna P K, Meiwes K J, Lindheim M, Prenzel J und Ulrich B 1982 Elementflüsse in Waldökosystemen im Solling-Datendokumentation-Göttinger Bodenk. Ber. 71, 267 p.
Matzner E and Ulrich B 1980 The transfer of chemical elements within a heath-ecosystem (Calluna vulgaris) in Northwest Germany. Z. Pflanzenernaehr. Bodenkd. 143, 666–678.
Miller H G and Miller J D 1980 Collection and retention of atmospheric pollutants by vegetation.In Ecological impact on acid precipitation, Eds. D Drablos and A Tollan. SNSF-project, Oslo, 33–40.
Minderman G and Leeflang K W F 1968 The amounts of drainage water and solutes from lysimeters. Plant and Soil 28, 61–80.
Reuss J O 1976 Chemical and biological relationships relevant to the ecological effects of acid rainfall.In Proc. first int. symposium on acid precipitation and the forest ecosystem. Eds. L S Dochinger and T A Seliga U.S.D.A. For. Serv. Gen. Tech. Rep. NE-23, 791–813.
Rosenqvist I T 1977 Sur jord-surt vann. Ingenørforlaget, Oslo, 123 p.
Sollins P, Grier C C, McCorison F M, Cromack K, Fogel R and Frederiksen R L 1980 The internal element cycles of and old-growth douglas-fir ecosystem in western Oregon. Ecol. Monographs 50, 261–285.
Stumm W and Morgan J J 1970 Aquatic Chemistry. Wiley-Interscience, New York, 583 p.
Tollan A (Ed.) 1977 Acid precipitation and some alternative sources as the cause of the acidifying of water sources. S.N.S.F. project. Oslo, Norway.
Ulrich B, Mayer R und Khanna P K 1979 Deposition von Luftverunreinigungen und ihre Auswirkungen in Waldökosystemen im Solling. J D Sauerländer's Verlag, Frankfurt am Main.
Van Beek C G E M and Van Breemen N 1973 The alkalinity of alkaline soils. J. Soil Sci. 24, 129–136.
Van Breemen N 1973 Soil forming processes in acid sulfate soils.In Dost H (Ed.) Acid Sulphate Soils, ILRI Publ. 18, 1973, Vol. I, 66–130, Wageningen, the Netherlands.
Van Breemen N 1975 Acidification and deacidification of coastal plant soils as a result of periodic flooding. Soil Sci. Soc. Am. Proc. 39, 1153–1157.
Van Breemen N 1976 Genesis and solution chemistry of acid sulfate soils in Thailand. Agric. Res. Rep. 848, Pudoc, Wageningen. Netherlands. 263 p.
Van Breemen N and Wielemaker W G 1974 Buffer intensities and equilibrium pH of minerals and soils. I. The contribution of minerals and aqueous carbonate to pH-buffering. Soil Sci. Soc. Am. Proc. 38, 55–60.
Van Breemen N and Wielemaker W G 1974 Buffer intensities and equilibrium pH of minerals and soils. Soil Sci. Soc. Am. Proc. 38, 61–66.
Van Breemen N, Burrough P A, Velthorst E J, Van Dobben H F, Toke de Wit, Ridder T B and Reynders H F R 1982 Soil acidification from atmospheric ammonium sulphate in forest canopy throughfall. Nature London 299, 548–550.
Van Breemen N, Van Grinsven J J M and Jordens E R 1983 H+ budgets and nitrogen transformations in woodland soils in the Netherlands influenced by high inputs of atmospheric ammonium sulfate. Proc. Int. Conf. Acid Precipitation—Origin and Effects VDI Düsseldorf, (In press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Breemen, N., Mulder, J. & Driscoll, C.T. Acidification and alkalinization of soils. Plant Soil 75, 283–308 (1983). https://doi.org/10.1007/BF02369968
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02369968