Abstract
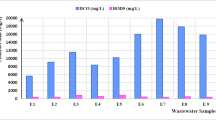
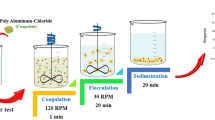
A coagulation treatment study was conducted using both natural (sago and potato flour) and commercial (poly aluminum chloride and aluminum sulfate) coagulants in semiconductor wastewater. The effects for settling time and dosage of the coagulants as well as their interactions on the reduction of chemical oxygen demand (COD) and turbidity were investigated using a three level factorial design, Response Surface Methodology (RSM). Sago concentration showed more influence on the COD and turbidity reduction than settling time, with concentrations lower than 1.5 g L−1 giving the better reduction. The interaction of settling time and concentration on the COD and turbidity were observed when using potato starch. Concentrations higher than 1.5 g L−1 potato starch reduced the COD and turbidity better. The polyaluminium chloride and ammonium sulphate revealed that lower concentrations (0.02–1.0 g L−1) and longer settling time (30–60 min) gave the greatest reduction in COD and turbidity.


Similar content being viewed by others
References
Aguilar, M. I., Saez, J., Llorens, M., Soler, A., & Ortuno, J. F. (2003). Microscopic observations of particle reduction in slaughterhouse wastewater by coagulation-flocculation using ferric sulphate as coagulant and different coagulant aids. Water Research, 37, 2233–2241.
Bratskaya, S., Schwarz, S., Liebert, T., & Heinze, T. (2005). Starch derivatives of high degree of functionalization 10. Flocculation of kaolin dispersions. Colloids and Surfaces. A: Physicochemical and Engineering Aspects, 254, 75–80.
Hollingsworth, J., Sierra-Alvarez, R., Zhou, M., Ogden, K. L., & Field, J. A. (2005). Anaerobic biodegradability and methanogenic toxicity of key constituents in copper chemical mechanical planarization effluents of the semiconductor industry. Chemosphere, 59, 1219–1228.
Lai, C. L., & Lin, S. H. (2003). Electrocoagulation of chemical mechanical polishing (CMP) wastewater from semiconductor fabrication. The Chemical Engineering Journal, 95, 205–211.
Lai, C. L., & Lin, S. H. (2004). Treatment of chemical mechanical polishing wastewater by electrocoagulation system performances and sludge settling characteristics. Chemosphere, 54, 235–242.
Lin, S. H., & Yang, C. R. (2004). Chemical and physical treatments of chemical mechanical polishing wastewater from semiconductor fabrication. Journal of Hazardous Materials, B108, 103–109.
Muyibi, S. A., & Evison, L. M. (1995). Optimizing physical parameters affecting coagulation of turbid water with Moringa oleifera seeds. Water Research, 29, 2689–2695.
Muyibi, S. A., & Okuofu, C. A. (1995). Coagulation of low turbidity of surface waters with moringa oleifera seeds. The International Journal of Environmental Studies, 48, 263–273.
Ndabigengesere, A., Narasiah, K. S., & Talbot, B. G. (1995). Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Research, 29(2), 703–710.
Nik Norulaini, N. A., Fatehah, M. O., Teng, T. T., Norli, I., & Sabrina, K. (2005). Induction of silica settling in wafer washing using pH adjustment, Seminar Kebangsaan Ke 4: Pengurusan Persekitaran 2005, Universiti Kebangsaan Malaysia, pp. 279–288.
O’ Melia, C. R. (1972). Coagulation and flocculation. In W. J. Weber (Ed.), Physicochemical process for water quality. New York: Wiley Interscience.
Rossini, M., Garrido, J. C., & Galluzzo, M. (1999). Optimization of the coagulant-flocculation treatment influence of rapid mix parameters. Water Research, 33(8), 1817–1826.
Tanyildizi, M. S., Őzer, D., & Elibol, M. (2005). Optimization of α-amylose production by Bacillus sp. using response surface methodology. Process Biochemistry, 40, 2291–2296.
Weber, W. J. (1972). Physicochemical processes fro water quality control. New York: Wiley Interscience.
Wu, M., Sun, D., & Tay, J. H. (2004). Process-to-process recycling of high-purity water from semiconductor wafer backgrinding wastes. Resources, Conservation and Recycling, 41, 119–132.
Acknowledgement
The authors are grateful to Advanced Micro Devices (M) Sdn. Bhd. (under the grant (304/PJJAUH/650309.A106) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Omar, F.M., Rahman, N.N.N.A. & Ahmad, A. COD Reduction in Semiconductor Wastewater by Natural and Commercialized Coagulants Using Response Surface Methodology. Water Air Soil Pollut 195, 345–352 (2008). https://doi.org/10.1007/s11270-008-9751-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9751-7