Abstract
Restoration of rich fens is commonly attempted through local-scale measures, such as removal of sod or blockage of ditches. However, regional-scale restoration measures, that aim to re-establish the original hydrology in which rich fens developed, might have a more long-lasting effect. We investigated the effect of local- and regional-scale restoration measures on a vulnerable rich fen in the Naardermeer nature reserve in the Netherlands. We compared water quality and vegetation composition of the fen before and after the restoration measures, almost 30 years apart. Overall rich fen species increased and although this indicates the desired increased supply of fresh mineral-rich groundwater to the fen, continued succession towards poor fen vegetation has not been prevented in the entire fen. Despite sod layer removal, we observed an increase in a Polytrichum-dominated vegetation in patches that are primarily fed by rainwater. Our findings confirm results from a previous study which showed that brackish palaeo-groundwater is still contributing substantially to the water balance of the fen, especially in periods of precipitation deficit. We conclude that the local- and regional-scale restoration measures have been successful in increasing the abundance of rich fen species in parts of the fen. However, considering the pressures of climate change and high atmospheric N-deposition on the fen, it is uncertain whether rich fen species can be sustained in quite nutrient-poor conditions in the future. Therefore, there is a need for continued management that keeps the nutrient-poor and mineral-rich conditions of the fen intact.
Similar content being viewed by others
Introduction
Rich fens are a wetland type with a particularly high value for nature conservation because of their species richness and their high proportion of endangered species (Bedford and Godwin 2003; Haapalehto et al. 2011). Rich fens are fed by surface water and/or groundwater that is generally high in minerals, hence the name ‘rich’ fen (Sjors and Gunnarsson 2002). The fact that many fen species are endangered is related to a number of adverse human impacts on fens. In Europe as well as in North America, the greater part of the original fen area has been reclaimed, primarily to create productive land for agriculture and forestry (Lamers et al. 2015). The remainder is often severely degraded due to human intervention in regional hydrology, such as groundwater extraction and drainage in nearby farmland, and is threatened by eutrophication (Lamers et al. 2015).
Hydrological processes greatly determine the biogeochemical conditions of fens (Schot et al. 2004). Wet fens have a lower nutrient availability than fens with periodically dryer conditions, as decomposition rates are lower in anoxic conditions (Fenner and Freeman 2011; Grootjans et al. 2006). The chemical composition of the inflowing water greatly determines the buffering capacity and nutrient availability of fens. For instance, the inflow of calcareous and Fe2+-rich water into fens facilitates the precipitation of dissolved phosphorus rendering it less available for plants (Griffioen et al. 2013; van der Grift et al. 2016). Low availability of phosphorus seems to be particularly beneficial for rich fen species (Kooijman et al. 2016; Wassen et al. 2005). Inflow of acidic rainwater or accumulation of rainwater affects plant composition both directly (via H+ toxicity) and indirectly (by influencing the availability of nutrients) (Lamers et al. 2015).
Acidification can occur naturally and drive succession that gradually transforms rich fens into transitional and poor fens, and finally into bogs (Joosten et al. 2017; Overbeck 1975; Succow and Jeschke 1986). For example, in the western part of the Netherlands this process has already occurred in turf ponds where the original rich fen peat had been excavated. Here, terrestrialization started again when a floating mat built up to a firm peat deposit that accumulated rainwater (Van Wirdum 1991; Westhoff 1971). Changes in hydrological processes due to human interference can speed up the succession towards poor fen and bog. Hydrological interferences that diminish water supply by surface water or upwelling groundwater cause the peat layer to dry out or result in increased infiltration of rainwater, leading to acidification of the top peat layer (Schot et al. 2004; Wassen et al. 1990). This also allows Sphagnum mosses to establish. Sphagnum produces acidic and poorly decomposing litter, causing further acidification and accumulation of peat. This positive feedback enforces succession towards poor fens that are less valuable from a nature conservation perspective (Barendregt et al. 1995; Granath et al. 2010; Wassen and Joosten 1996).
Restoration of rich fens often focuses on re-establishing the nutrient-poor and pH-neutral conditions, using local measures such as rewetting by blocking drainage ditches or increasing phreatic groundwater level (Bootsma et al. 2002). However, these restoration projects show variable degrees of success (Lamers et al. 2015; van Dijk et al. 2004; Zak and Gelbrecht 2007). For instance, if the supplied water lacks buffering capacity, rewetting alone is not sufficient to counteract acidification. Restoring the regional hydrology, i.e. the hydrology of the catchment in which the fen area is located, has been proposed as the most rigorous way of restoring the habitat quality of rich fens (Klimkowska et al. 2010). However, this involves large-scale, costly and time-consuming measures, whose effectiveness is unclear due to a lack of long-term monitoring data.
In the Naardermeer nature reserve in the Netherlands, we had the rare opportunity to test the effectiveness of local and regional restoration measures on a groundwater-fed fen. We compare detailed surveys of water quality and vegetation which were carried out in the fen before (1987) and after (2016) restoration measures were taken in the 1990s. The fen has been protected since 1904 and has been of interest to botanists since the 1930s (Barendregt et al. 1995). Despite the long-term status of protected nature reserve, the occurrence of rich fen species communities in the fen decreased in the period 1937–1987 (Barendregt et al. 1995). This shift has been attributed to a reduction in seepage of fresh, calcium-carbonate-rich groundwater (Wassen et al. 1989). In an attempt to restore the original hydrology of the area, groundwater extraction has been diminished in the regional goundwater recharge area Het Gooi. This has been predicted to have a positive effect on the supply of calcium-carbonate-rich groundwater. In addition, the sod layer was removed from some patches with a high abundance of Polytrichum and Sphagnum moss. Thick moss carpets prevent the establishment of vascular plants and isolate the fen from the inflow of groundwater (Bootsma et al. 2002; Lamers et al. 2002). Finally, local ditches in the fen were re-excavated to drain excess rainwater. The aim of the present study is to assess the impact of the local and regional restoration measures on the hydrology and vegetation of the fen.
Methods
Site description
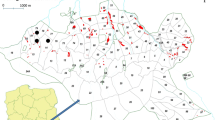
The Naardermeer nature reserve is a 700 ha wetland consisting of lakes, marshes, fens and forest. It is situated in the centre of the Netherlands, about 20 km southeast of Amsterdam (52°17′ N and 5°08′ W, Fig. 1a), and it has been a protected area for over 100 years. The Naardermeer is a polder with a fixed water level of 1.0 m below mean sea level (m.s.l.). It loses water towards surrounding polders with an even lower water level and receives water via upwelling groundwater originating from the elevated ice-pushed ridge Het Gooi (Fig. 1b). The fen that we studied is located on the east side of Naardermeer, next to Lake Bovenste Blik (Fig. 1c). Over the last 80 years, several studies have been carried out in this fen (see Barendregt et al. 1995; Bouman 2013; Wassen et al. 1989). In the late nineteenth century the fen probably started to grow at the edge of the lake in which Characeae flourished in abundance and where Phragmites australis and Typha spec. grew at its shores (Bouman 2013). The wet reedland that developed was mown regularly for reed harvesting in winter until around 1950 and since then irregularly until 1973. After 1973, a strip of approximately 100 m wide, adjacent to the lake, was mown annually in August, whereas further away from the lake the fen developed into a woodland with Alnus glutinosa and Betula pubescens (Bouman 2007, 2013). Barendregt et al. (1995) studied original vegetation descriptions and reconstructed the history of the fen from 1937; they concluded that succession had taken place. A loosely structured peat layer was present until 1972, with small pools which contained an abundance of rich fen species. In the 1980s presence and abundance of rich fen species was lower, and species of acid reed marshes had appeared, such as Sphagnum species, Polytrichum commune, Aulacomnium palustre and Carex curta. Peat depth is c. 80 cm (Wassen et al 1989).
In the fen, both fresh and brackish palaeo-groundwater comes to the surface. The brackish groundwater comes from Holocene sea water intrusions which entered the aquifer from above by density flow (Post et al. 2003; van Loon et al. 2009). The fresh groundwater that seeps up in the fen comes from the adjacent ridge Het Gooi (Fig. 1b). In Het Gooi, groundwater has been extracted for the production of drinking water since the 1920s, with a peak amount of about 17 million m3 per year in the 1970s. This has reduced the discharge of groundwater into the fen area (Barendregt et al. 1995). As part of a larger restoration plan for the whole Naardermeer nature reserve, measures have been taken to increase the supply of fresh groundwater, aiming to bring back the original hydrology of the reserve. In Het Gooi, drinking water extraction has gradually been decreased to this end. A nearby pumping station was closed permanently, and extraction has been diminished in other pumping stations, leading to a reduction of drinking water extraction in 1998 by approximately half the original extraction. Two pumping stations located in the part of Het Gooi that potentially supply the Naardermeer with discharging groundwater are currently only extracting water for industrial purposes (1 million m3/year). In addition, water tables have been raised in a buffer zone adjacent to the nature reserve. Finally, measures have been taken to increase the infiltration of rainwater in the urban areas of Het Gooi, all with the aim of recharging the groundwater aquifer that discharges in the Naardermeer (Boosten 2006).
In the fen annual mowing has been practised since 1977, and the hay is subsequently removed. In 1994, two overgrown ditches were re-excavated, and the sod layer was removed in two patches of dense Polytrichum moss with a total surface area of 2100 m2 (Fig. 1c) (Bouman 2013).
Field study and lab analysis
This study combines data collected in the 1980s from Wassen et al. (1989), who carried out a detailed study in the fen in 1987, and (Schot, unpubl.), who sampled phreatic water in the fen in 1986, with data from recent field studies in 2016 and 2018. Both 1980s and the recent study sampled phreatic groundwater and recorded plant species composition of vegetation and are similar in design. However, the hydrological mapping of the study area is more detailed for 2016 than for 1987 and in 2016 we also sampled aboveground vegetation for analysis of N and P.
The study area is 1.8 ha and is divided into 182 plots of 10 by 10 m. Effort was taken to align the plots in exactly the same way as in 1987. In 1987 and 2016, the abundance of 77 plant species was recorded, using a 6-point Tansley scale. Electrical conductivity (EC25) and depth of the water tables relative to the peat surface were measured in the middle of each plot in shallow pits with a depth of approximately 15 cm. In 1987, these measurements were taken between 2 June and 2 July. To account for short-term variations, EC and water tables were measured twice in 2016, six weeks apart, on 17 May and 30 June. To study the influence of rainwater in the study area in more detail, EC and water tables were recorded again after an exceptional period of drought in 40 plots at a depth of approximately 15 cm on 15 August 2018. Net precipitation in the month preceding the sampling was 120 mm, 46 mm, 149 mm, 37 mm for June 1987, May 2016, June 2016 and August 2018, respectively (weather station Laren, approximately 7 km southeast of the study area) (KNMI 2019a).
More information on the water quality of the study area was obtained from 40 groundwater piezometers that were evenly distributed over the area. These had a filter depth of 40–50 cm below the surface, were present only during the field survey in 2016 and were sampled twice during this period (on 20 May and 5 July 2016). Prior to sampling, piezometers were emptied and left to refill again before measuring on the same day. In the piezometers, pH, temperature and EC were measured using a portable field metre (Eijkelkamp Aquaread AP-700 Aquaprobe), and the water table below the peat surface was measured. Water samples were taken from the piezometers, the ditches and the adjacent lake. HCO3− concentration was measured on the same day using a titration method (Hach Alkalinity Test Kit, Colorado, US). Samples were taken to the lab and centrifuged and acidified before analysis of major ions Mg2+, Fe2+/3+, Na+, Cl−, Ca2+ and K+ on ICP-MS. Additionally, we analysed 8 samples that were taken from the lake and the two ditches in the fen (Fig. 1). Data on Ca2+ and Cl− concentration in the study area prior to the restoration measures were obtained from a detailed hydrological study in 1986 (Schot, unpubl.).
In 13 randomly selected 20 by 20 cm subplots aboveground, biomass was cut near the soil surface and collected in paper bags to examine nutrient limitation in the fen. Samples were dried at 70˚C and dry weight was determined. N and P concentration was measured after Kjeldahl digestion. N:P ratios were used as a proxy for nutrient limitation sensu Olde Venterink et al. (2003). N:P ratios ≤ 13.5 indicate N limitation (relative to P) and ratios > 16 indicate P limitation (relative to N). Values between 13.5 and 16 indicate N and P co-limitation or no clear limitation by N or P.
Statistical analysis
The spatial distribution of EC in the study area in June 1987, May 2016, June 2016 and August 2018 was presented on maps. Between the measuring points, the data were linearly interpolated. To examine short and long-term changes in ion richness and Ca2+ and Cl− concentration, scatterplots were produced in which all the sampling periods were plotted against each other (EC, Ca2+ and Cl−). Regression lines between sampling periods were fitted with linear least squares regression.
We calculated the mean depth of the water tables and groundwater quality variables (EC, pH, Na+, Ca2+, Mg2+, K+, Cl−, HCO3−, Fe2+/3+) measured in May and July before using them in the further analysis. All variables except water table were ln-transformed to obtain an approximate normal distribution and homoscedasticity of errors. The variation in water quality was explored using Principal Component Analysis (PCA). Variables were normalised prior to performing the PCA. To show the spatial variation in water quality in the study area, the sample scores of the first principal component were displayed on a map using linear interpolation between the 40 sample points.
Additionally, we used Ellenberg indicator values (Ellenberg et al. 1992) for describing the environmental conditions experienced by the plant species. On an ordinal nine-point or twelve-point scale, these values indicate under which field conditions a species is most likely to be found. We used Ellenberg indicator values for moisture (F – 12-point scale, 1 = dry, 12 = wet); acidity (R − 9-point scale, 1 = acid, 9 = alkaline); and nutrients (N − 9-point scale, 1 = nutrient-poor, 9 = nutrient-rich).
Changes in the abundance of species were tested using a paired Wilcoxon test. Sites were clustered using Ward’s clustering method, based on the abundances of all species recorded in 1987 and 2016. Ward’s clustering aims at minimizing the within-group variance. The Euclidean distance was used as distance measure. Six clusters were identified based on dissimilarity levels and evaluation of species presence and abundance, and they were given a vegetation type name, using expert judgement. The spatial distribution of the vegetation types in 1987 and 2016 was displayed on maps, and differences in the abundance of species between 1987 and 2016 were tested using a paired Wilcoxon test. Lastly, differences in water quality and N:P ratio between the vegetation types were examined by performing a one-way ANOVA.
All analyses were carried out in R-studio (R Core Team 2019). The packages used for analysis were vegan, cluster and akima.
Results
Water quality
A wide range of EC values were found in the study area; the lowest measured value was 56 µS/cm (June 2016) and the highest was 2040 µS/cm (August 2018). Most measurements were between 170 and 610 µS/cm. In 1987, a clear zonation of increasing EC towards the lake was found (Fig. 2d). In the southwest and northwest corners of the study area, water with very high EC (> 1000 µS/cm) was observed, indicating brackish influences. This brackish water is not stemming from lake water, since lake water only had an EC of 400–600 µS/cm and no flooding was observed (Wassen et al. 1989). Water with low EC (< 200 µS/cm), indicating rainwater, was found in the zone adjacent to the forest edge (see also Wassen et al. 1989). This clear zonation was no longer present in 2016 or in 2018. In May and June 2016 (Fig. 2b, c), the zone of low EC extended from the edge of the forest towards the lake at the northern half of the study area, but in the southern half of the study area, higher EC values were also found at the forest edge. This pattern was the same in 2018, although it differed with respect to the areas where brackish water seeps up (Fig. 2a). The area of high EC on the southwest side of the study area seemed to have disappeared in 2016, but then re-appeared in 2018. The summer of 2018 was an exceptionally dry period with a precipitation deficit of approximately 300 mm over the month prior to sampling (KNMI 2019b). As in 1987, neither in 2016 nor in 2018 flooding with lake water occurred and lake water had an EC of 570–875 µS/cm in these years, indicating that the zones with high EC in the fen (> 1000 µS/cm; Fig. 2a, c) cannot be attributed to lake water.
In general, EC values were lower in 2016 than in 1987 (Fig. 3a, b; slope 0.41 and 0.35, respectively). The EC values of 1987 were similar to those of 2018 (Fig. 3d, slope 1.05). The differences in EC between May and June 2016 (Fig. 3c) were small and can be explained by the amount of net precipitation in these months (46 mm in May, 149 mm in June) (measuring station Laren, KNMI 2019a). In 2016, more values were concentrated at low to intermediate EC values than in 1987 (between approximately 100 and 400 µS/cm) and, although we encountered some outliers with values above 1000 µS/cm, the overall range in 2016 was narrower than in 1987 (Fig. 3a, b).
Comparison of water quality variables that were measured in June 1987, May 2016, June 2016 and August 2018. a–f Electrical conductivity (EC in µS/cm) at 15 cm depth, N = 182 in 1987 and 2016, N = 40 in 2018. g–i Ca2+-concentration (mg/l) measured in groundwater piezometers, N = 14 in 1987 and N = 40 in 2016. j–l Cl−-concentration (mg/l) measured in groundwater piezometers, N = 14 in 1987 and N = 40 in 2016
Ca2+ concentration did not change much between 1986 and 2016 (Fig. 3g, h); concentrations ranged from almost zero to about 120 mg/l, indicating that a full range of almost rainwater to calcareous water occurred in the fen. Similar to EC, there was an overall decrease in Cl− concentration between 1986 and 2016. Very high values (> 200 mg/l Cl−) were measured in 1986, but in 2016 almost all Cl− concentrations measured were below 200 mg/l (Fig. 3j, l). Concentrations in Ca2+ and Cl− in May 2016 were quite similar to those in June 2016 (Fig. 3i, l), indicating no great differences occurred in two months in 2016.
About half of the proportion of the variance in water quality parameters is explained by the first PCA component (54%; Table 1). The first PCA component comprises the general increase in all ion concentrations except for K+ and Fe2+/3+, the second component expresses differences in K+ concentration, and the third component consists of opposing variation in water table (note that water tables are expressed in cm relative to the surface, i.e. positive values indicate inundated conditions and negative values indicate water tables below the surface). However, the latter two axes explain only 13% and 10% of the variance, respectively. Water table differences are apparently not clearly related to differences in water quality, although the first component shows that sites with higher concentrations of solutes are wetter (Table 1). Figure 4 presents the spatial distribution of water quality over the fen area in 2016, based on the sample scores on the first principal component. It shows a pattern that is comparable to the EC maps (Fig. 2b, c), indicating that EC reflects richness in major ions such as Cl−, Na+, Mg2+, Ca2+ and HCO3−.
Interpolated map of the sample scores on the first principal component (see Table 1). Study grid is indicated by grey lines, groundwater piezometers (N = 40) are indicated by circles. Shaded plots lie outside interpolation area
Plant species occurrences
Table 2 lists all the species that were mapped in 1987 and 2016. From this table the following trends emerge:
-
1.
Some low-stature rich fen species have increased in abundance (Carex diandra, Carex nigra, Dactylorhiza majalis, Epipactis palustris, Juncus acutiflorus, Lotus uliginosus and Pedicularis palustris) or appeared in the period 1987–2016 (Carex echinata, Carex oederi subsp. oedocarpa, Liparis loeselii and Succisa pratensis), whereas Carex curta, Galium palustre, Potentilla palustris, Stellaria palustris and Salix repens declined and Valeriana dioica did not change in abundance.
-
2.
Many of the taller-growing species of rich fens have decreased in abundance (Carex riparia, Iris pseudacorus, Lycopus europaeus, Lysimachia vulgaris, Lythrum salicaria, Phragmites australis, Rumex hydrolapathum, Solanum dulcamare, Thelypteris palustris, Typha angustifolia and Valeriana officinalis) whereas Carex paniculata, Eupatorium cannabinum and Juncus subnodulosus have increased.
-
3.
Many species of moist meadows have decreased in abundance (Calamagrostis canescens, Caltha palustris, Cardamine pratensis, Holcus lanatus, Holcus mollis and Lychnis flos-cuculi) whereas only two species of moist meadows have increased in abundance (Anthoxanthum odoratum and Rhinanthus serotinus).
-
4.
Calliergonella sp. have decreased in abundance, Sphagnum sp. have shown no change in abundance and Polytrichum sp. have increased in abundance.
Inspection of Ellenberg indicator values for moisture, acidity and nutrients revealed substantial variation amongst the occurring species in the indicator values for acidity and nutrients, but not for moisture. Median and mean values showed that species that increased in abundance between 1987 and 2016 generally had lower values for acidity and nutrients than species which decreased or species that showed no change in abundance, whereas the moisture indication values remained the same.
Vegetation types
Clustering revealed six vegetation types (Table 3) that were separated into two main types at a high dissimilarity level (28, Online Resource 1) on the basis of high presence of Calliergonella sp., Calamagrostis canescens, Iris pseudacorus and other rich fen species (type 1 referred to as rich fen reedland) versus lower abundance of rich fen species and presence of Eriophorum angustifolium, Drosera rotundifolia, Anthoxanthum odoratum and other poor fen species (type 2 referred to as poor fen reedland). The rich fen reedland is subdivided into a Thelypteris-Sphagnum intermediate fen type (1A) and a Thelypteris-Calliergonella rich fen type (1B) at a dissimilarity level of 14 (Online Resource 1), the latter having a higher presence of tall growing rich fen species such as Carex riparia, Rumex hydrolapathum and Lycopus europaeus than the former. Type 1B is subdivided into two subtypes. 1B1 has the highest abundance of Thelypteris palustris and Calliergonella sp. and the highest presence of tall growing species such as Angelica sylvestris, Valeriana officinalis and Typha angustifolia of all 6 types (referred to as the Thelypteris-Calliergonella subtype). 1B2 is dense reedland with a high abundance of Phragmites australis and compared to 1B1 higher presence of tall growing species such as Rumex hydrolapathum and Carex acutiformis and species such as Berula erecta, Myosotis palustris, Eupatorium cannabinum and Mentha aquatica. This subtype (1B2) has many species in common with type 2A, but with a lower presence. Type 1B2 is referred to as Thelypteris-Carex acutiformis subtype.
Type 2A and 2B are separated at a quite high dissimilarity level of 23 (Online Resource 1). Type 2A is a Sphagnum reedland but with high presence of rich fen elements such as Juncus subnodulosus, Pedicularis palustris, Carex oederi subsp. oedocarpa and Carex paniculata, but also poor fen elements such as Eriophorum angustifolium, Drosera rotundifolia, Carex echinate and Hydrocotyle vulgaris (referred to as Sphagnum poor fen). It is subdivided into two subtypes, mainly based on a higher abundance of for example Carex diandra, Carex paniculata and Thelypteris palustris and higher presence of Epipactis palustris, Liparis loeselii and Utricularia minor in subtype 2A2, which is referred to as Carex diandra poor fen. Subtype 2A1 is characterized by a high presence of Drosera rotundifolia and Juncus acutifloris which is referred to as Drosera poor fen. Type 2B substantially differs from the other communities; it is a relatively species-poor community in which most rich fen species are absent or have low presence (Online Resource 1) and which is dominated by mosses (Polytrichum and Sphagnum spec. and Aulacomnium palustre). It is referred to as Polytrichum poor fen.
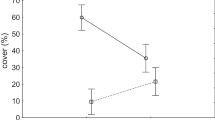
Two of the six vegetation types were present in both 1987 and 2016. One of these is the dense reedland Thelypteris-Carex acutiformis subtype (1B2). This vegetation type extended in cover from 22 cells in 1987 to 45 cells in 2016 (Fig. 5). In 1987, this type was only found along the lake, but in 2016 it was also found along the two ditches that were re-excavated in 1994. The second type that was present in both years is the Polytrichum poor fen (2B), the relatively species-poor community dominated by Polytrichum moss. The area covered by this vegetation type extended from 23 cells in 1987 to 39 cells in 2016, mainly in the northeast corner of the study area (Fig. 5). This expansion came at the expense of the area covered by the Thelypteris-Sphagnum intermediate fen (1A). In 1987, the latter type covered a large patch along the forest edge that expanded to the centre of the fen. In 2016 it almost disappeared and was replaced by the Thelypteris-Carex acutiformis fen (1B2) and the Drosera, Carex diandra and Polytrichum poor fens (2A1, 2A2, 2B).
Large areas covered by rich fen and intermediate fen in 1987, primarily vegetation type 1A (Thelypteris-Sphagnum intermediate fen) and 1B1 (Thelypteris-Calliergonella rich fen), completely disappeared between 1987 and 2016. The Thelypteris-Calliergonella rich fen (1B1) was abundant in the belt behind the lake side vegetation in 1987 and was replaced in 2016 by poor fen, especially the Drosera (2A1) and the Carex diandra poor fen (2A2), as well as the Thelypteris-Carex acutiformis fen (1B2), the latter mainly along the northern re-excavated ditch.
Water quality of the vegetation types
Figure 6 shows the variation in EC of the shallow peat water between the vegetation types. Generally, rich fens have a higher EC than poor fens, and the Thelypteris-Sphagnum intermediate fen (1A) has a significantly lower EC than the rich fens (Thelypteris-Calliergonella fen (1B1) and Thelypteris-Carex acutiformis fen (1B2)). The highest EC values and the largest variation in EC were found in the Thelypteris-Calliergonella fen (1B1) and the Thelypteris-Carex acutiformis fen (1B2). The Polytrichum poor fen (2B) has the lowest EC (< 200 µS/cm), although this is not significantly lower than the Drosera fen (2A1) and the Thelypteris-Sphagnum intermediate fen (1A). The Carex diandra fen (2A2) stands out amongst the other poor fens with significantly higher EC values but still significantly lower EC values than the rich fen types (Thelypteris-Calliergonella fen and Thelypteris-Carex acutiformis fen (1B1 and 1B2)). In the Carex diandra fen, the EC is between 200 and 600 µS/cm.
Boxplots of EC in shallow peat water (< 15 cm depth) in the vegetation types (values measured in 1987 and 2016). 1A1 N = 50, 1A2 N = 27, 1B1 N = 62, 1B2 N = 67, 2A1 N = 59, 2A N = 37, and 2B N = 39. Significant differences (p < 0.05) between all types are indicated by letters, the significant difference between the rich and poor type together is indicated by asterisks (p < 0.001)
Figure 7 presents boxplots of the water table and water quality parameters of vegetation types that were present and sampled in 2016 (1B2, 2A1, 2A2 and 2B). The Thelypteris-Carex acutiformis fen (1B2) generally has the highest concentration in all major ions, except Fe2+/3+ and K+. Fe2+/3+ concentration does not differ between the vegetation types. The Polytrichum poor fen has the highest variability in water table and the highest K+ concentration, but generally has a low concentration of the major ions and a low pH. The Drosera fen (2A1) has values as low as the Polytrichum poor fen (2B) for Na+, Ca2+, Cl− and Mg2+. The Carex diandra fen (2A2) does not significantly differ from the Thelypteris-Carex acutiformis fen (1B2) but has intermediate concentrations for Na+, Ca2+, Cl, HCO3− and Mg2+ in between the Thelypteris-Carex acutiformis fen (1B2) on one hand and the Drosera fen (2A1) and Polytrichum poor fen (2B) on the other hand.
N:P in vegetation
Figure 8 presents N and P concentrations and the N:P ratio in the aboveground plant material for the vegetation types encountered in 2016. N concentrations did not differ significantly between the vegetation types. P concentrations were highest in the Polytrichum poor fen (2B), although not significantly higher than in the Thelypteris-Carex acutiformis fen (1B2). Most N:P ratios were well above 14.5, indicating P-limited conditions (11 out of 13 plots); only the Polytrichum poor fen had ratios indicating N-limited conditions. The N:P ratio was significantly higher in the Carex diandra fen (2A2) than in the other three communities; the ratios of > 30 measured in this fen type indicate strongly P-limited conditions.
Discussion
The setup of the current study was designed to match as well as possible the detailed hydrological and botanical study carried out in the fen area in 1987 (Wassen et al. 1989). This offers the unique opportunity of examining the effectiveness of the local and regional restoration measures targeting at preserving rich fen vegetation in the area.
Before discussing the observed changes in species composition and water quality, we discuss the vegetation types of the fen studied in the context of European fen syntaxonomy (Peterka et al., 2017). Only the fen type that we referred to as Polytrichum poor fen (type 2B) shows similarities to an alliance classified by Peterka et al. (2017), i.e. the Sphagno-Caricon canescentis. This is an alliance with low species richness and low mineral supply, which can be seen as an initial stage of the fen-bog transition. This alliance has been described as a transition to initial stages of ombrotrophic mires (bogs) illustrated by Sphagnum species, but nevertheless still with a minerotrophic water regime indicated by species that do not enter pristine bogs, e.g. Agrostis canina, Carex echinata, C. nigra, Potentilla palustris and Viola palustris (Peterka et al. 2017). All these species were present in our fen, exemplifying that part of our fen is in a transitional state in which both calcareous groundwater and rainwater are present in the shallow peat layer. Inspection of Ellenberg indicator values for moisture, acidity and nutrients indicated that the moisture status of the fen has not changed, whereas in general conditions in the fen have become slightly more acidic and nutrient-poorer.
We identified quite substantial changes both in hydrological and water quality variables and in vegetation of the fen. The clear pattern of increasing ion richness from forest to lake that was observed in 1987 is no longer present in recent years, nor was it reflected in the zonation of vegetation types. There is a general increase in low-stature rich fen species (e.g. Carex diandra, Dactylorhiza majalis, Epipactis palustris and Pedicularis palustris). Moreover, it is noteworthy that there was a re-appearance of some valuable species from a nature conversation perspective (e.g. Liparis loeselii, Succisa pratensis and Utricularia minor) (Barendregt et al. 1995). The strong phosphorus limitation of the fen, especially in the Carex diandra fen (2A2), shows that phosphorus limitation may be a causal factor for the increase in these rich fen species. The restored inflow of calcium-carbonate-rich fresh groundwater may have decreased the nutrient availability by preventing acidification and desiccation, and by binding phosphate to base cations (Granath et al. 2010; Lamers et al. 2002). The pH values between 6 and 7 and high HCO3− concentrations in the Carex diandra fen and the Thelypteris-Carex acutiformis fen support this conclusion. However, nutrient availability may also have decreased due to long-term cutting of vegetation and the removal of the hay, which has been done annually in our fen since the 1970s (Bouman 2013). This may well have caused a gradual removal of nutrients, especially phosphorus (Koerselman and Verhoeven 1992). Therefore, the nutrient status in the fen cannot be directly linked to the hydrological restoration measures only.
Although it is probable that the flow of fresh groundwater to the fen has increased, this is not sufficient to realise the desired development towards rich fen in the entire fen. The increase in cover of poor fen vegetation with a high Polytrichum abundance (Polytrichum poor fen) is indicative of an increased influence of rainwater. This is also the type in which the lowest pH and HCO3− concentrations have been found. The vigorous spread of this vegetation type came primarily at the cost of the Thelypteris-Sphagnum intermediate fen along the forest edge. Polytrichum can quickly establish in acidic, often Sphagnum-covered, vegetation (Paulissen et al. 2004). The buffering capacity in this part of the fen may have been insufficiently restored to prevent acidification and further succession to poor fen. Since the spread of Polytrichum poor fen is outside the area where the sod has been removed this may indicate that restoring the regional hydrology without local measures such as sod removal is insufficient to halt succession towards Polytrichum poor fen. This succession could also have been enforced by the high atmospheric deposition in this part of the Netherlands. The increased ammonium concentration caused by atmospheric nitrogen deposition negatively affects brown mosses, whilst Sphagnum and Polytrichum mosses are tolerant to it (Paulissen et al. 2004).
Potentially, the removal of the sod layer can accommodate restoration of the rich fen type in the Polytrichum poor fen. However, sod layer removal is only effective if groundwater seepage into the root zone is sufficient (Bootsma et al. 2002). In 1994, an approximately 20 cm deep sod layer was removed over a total surface area of 2100 m2 (12% of the study area), which was covered in dense Polytrichum moss (Bouman 2013). In 2016, these patches were mostly occupied by the Carex diandra fen, with a circum-neutral pH and high alkalinity, indicating that sod layer removal was beneficial for restoring the upward seepage into the root zone. The ditches that were dug out again after 1987 are clearly recognizable in the distribution pattern of the Thelypteris-Carex acutiformis fen and partly also in the EC. Apparently, this local intervention also enhanced the supply of base-rich water. This is probably upwelling groundwater and not inflowing lake water, since the water tables in the fen are—as far as we know—always higher than the water levels in the lake.
The intention of diminishing the drinking water extraction was to reinforce the regional hydrological freshwater system that supplies the fen with upwelling groundwater. This was expected to diminish the infiltration of rainwater into the fen and to push the brackish groundwater that seeped up in the fen westward. However, comparable high EC values were found in the Thelypteris-Calliergonella fen (1B1, present in 1987 only) and the Thelypteris-Carex acutiformis fen (1B2, found mainly in 2016). This clearly results from the inflow of brackish groundwater that seeps up in the ditches and the plots adjacent to the lake (see Online Resource 2 for comparison of ditch and lake water).
Apparently, the upward seepage of regional fresh groundwater is not sufficient to push away the seepage of brackish palaeo-groundwater from the fen, especially in periods of drought, such as in August 2018. The high EC values that we measured in this dry spell of 2018 (2040 µS/cm), when there was a negligible effect of rainwater, clearly show that brackish groundwater was seeping into the fen. This was also the case in May 2016 although to a minor extent. Furthermore, van Loon et al. (2009) showed in a modelling study corroborated with empirical data that in 2006, during periods of precipitation deficit, brackish groundwater were flowing into the fen. Van Loon et al. (2009) also suggested that fresh groundwater flow is intercepted by ditches in close-by agricultural land, thereby redirecting it before it can flow to the fen. However, we stress that the groundwater that seeps up in the major part of the studied fen comes from a regional freshwater system and is still seeping up into the fen in dry periods. Nonetheless, the flux is probably less in dry periods, allowing brackish groundwater to gain influence in the fen. Still, this brackish influence occurs only on the lake side of the ditches, whereas the greater part of the fen is still supplied by regional fresh groundwater as indicated by EC values up to 600 µS/cm.
Our study shows the complexity of restoring rich fens to their original state and preventing them from rapid succession into poor fen. Even though this project has attempted to preserve the vulnerable rich fen vegetation of the Naardermeer, it should be kept in mind that there are several factors that hamper the sustainability of mesotrophic rich fens. The high atmospheric nitrogen deposition in this part of the Netherlands, that apart from eutrophication also causes additional acidification, and the increasingly frequent dry summers that are expected to come along with climate change, mean that fens must be resilient enough to cope with these stressors. Both of these trends may favour the growth of Polytrichum at the cost of rich fen species. This does not mean that there is no way of preserving the high value of this wetland type (Klimkowska et al. 2010; Kotowski et al. 2016). However, management that is aimed at keeping pH-neutral and nutrient-poor conditions of rich fens intact is crucial, even though it comes at a high price.
References
Akima H, Gebhardt A (2016) Akima: interpolation of irregularly and regularly spaced data. Version: 0.6-2.
Barendregt A, Wassen MJ, Schot PP (1995) Hydrological systems beyond a nature-reserve, the major problem in wetland conservation of Naardermeer (the Netherlands). Biol Conserv 72:393–405. https://doi.org/10.1016/0006-3207(94)00101-U
Bedford BL, Godwin KS (2003) Fens of the United States: Distribution, characteristics, and scientific connection versus legal isolation. Wetlands 23:608–629. https://doi.org/10.1672/0277-5212(2003)023[0608:Fotusd]2.0.Co;2
Boosten A (2006) Meer, Meer: 13 jaar Herstelplan Naardermeer. 's-Gravenland
Bootsma MC, van den Broek T, Barendregt A, Beltman B (2002) Rehabilitation of acidified floating fens by addition of buffered surface water. Restor Ecol 10:112–121. https://doi.org/10.1046/j.1526-100X.2002.10112.x
Bouman AC (2007) Naardermeer, beheerverslag 1936–2000. Rapport Vereniging Natuurmonumenten
Bouman AC (2013) Het Naardermeer: Twintig jaar monitoring in de oeverlanden aan de oostzijde van Bovenste Blik. Rapport Vereniging Natuurmonumenten
Ellenberg H, Weber H, Düll R, Wirth V, Werner W, Paulissen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa, 2nd edn. Gotze, Göttingen
Fenner N, Freeman C (2011) Drought-induced carbon loss in peatlands. Nat Geosci 4:895–900. https://doi.org/10.1038/Ngeo1323
Granath G, Strengbom J, Rydin H (2010) Rapid ecosystem shifts in peatlands: linking plant physiology and succession. Ecology 91:3047–3056. https://doi.org/10.1890/09-2267.1
Griffioen J, Vermooten S, Janssen G (2013) Geochemical and palaeohydrological controls on the composition of shallow groundwater in the Netherlands. Appl Geochem 39:129–149. https://doi.org/10.1016/j.apgeochem.2013.10.005
Grootjans AP, Adema EB, Bleuten W, Joostn H, Madaras M, Janakova M (2006) Hydrological landscape settings of base-rich fen mires and fen meadows: an overview. Appl Veg Sci 9:175–184. https://doi.org/10.1658/1402-2001(2006)9[175:Hlsobf]2.0.Co;2
Haapalehto TO, Vasander H, Jauhiainen S, Tahvanainen T, Kotiaho JS (2011) The effects of peatland restoration on water-table depth, elemental concentrations, and vegetation: 10 years of changes. Restor Ecol 19:587–598. https://doi.org/10.1111/j.1526-100X.2010.00704.x
Joosten H, Moen A, Couwenberg J, Tanneberger F (2017) Mire diversity in Europe: mire and peatland types. Mires and Peatlands of Europe: status, distribution and conservation. Schweizerbart Science Publishers, Stuttgart, pp 5–64
Klimkowska A, Van Diggelen R, Grootjans AP, Kotowski W (2010) Prospects for fen meadow restoration on severely degraded fens. Perspect Plant Ecol Evol Syst 12:245–255. https://doi.org/10.1016/j.ppees.2010.02.004
KNMI (2019a) Dagwaarden neerslagstations. https://www.knmi.nl/nederland-nu/klimatologie/monv/reeksen. Accessed Jul 14, 2019
KNMI (2019b) Neerslagtekort in Nederland. https://www.knmi.nl/nederland-nu/klimatologie/geografische-overzichten/neerslagtekort_droogte. Accessed Jul 14, 2019
Koerselman W, Verhoeven JTA (1992) Nutrient dynamics in mires of various trophic status: nutrient inputs and outputs and the internal nutrient cycle. Fens and bogs in the Netherlands. Springer, Berlin, pp 397–432
Kooijman AM, Cusell C, Mettrop IS, Lamers LPM, Middleton B (2016) Recovery of target bryophytes in floating rich fens after 25 yr of inundation by base-rich surface water with lower nutrient contents. Appl Veg Sci 19:53–65. https://doi.org/10.1111/avsc.12197
Kotowski W et al (2016) Restoration of temperate fens: matching strategies with site potential. Peatland Restor Ecosyst Serv. https://doi.org/10.1017/cbo9781139177788.011
Lamers LP et al (2015) Ecological restoration of rich fens in Europe and North America: from trial and error to an evidence-based approach. Biol Rev Camb Philos Soc 90:182–203. https://doi.org/10.1111/brv.12102
Lamers LPM, Smolders AJP, Roelofs JGM (2002) The restoration of fens in the Netherlands. Hydrobiologia 478:107–130. https://doi.org/10.1023/A:1021022529475
Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2018) Cluster: cluster analysis basics and extensions Version: 2.0.7-1
Oksanen J, F. et al. (2019) Vegan: Community Ecology Package Version: 2.5-5
Olde Venterink H, Wassen MJ, Verkroost AWM, De Ruiter PC (2003) Species richness–productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 84:2191–2199. https://doi.org/10.1890/01-0639
Overbeck F (1975) Botanisch-geologische Moorkunde unter besonderer Berucksichtigung der Moore Nordwestdeutschlandsals Quellen zur Vegetations-, Klima-und Siedlungsgeschichte. Karl Wacholtz Verlag, Neumünster
Paulissen MPCP, Van Der Ven PJM, Dees AJ, Bobbink R (2004) Differential effects of nitrate and ammonium on three fen bryophyte species in relation to pollutant nitrogen input. New Phytol 164:451–458. https://doi.org/10.1111/j.1469-8137.2004.01196.x
Peterka T et al (2017) Formalized classification of European fen vegetation at the alliance level. Appl Veg Sci 20:124–142. https://doi.org/10.1111/avsc.12271
Post VEA, Van der Plicht H, Meijer HAJ (2003) The origin of brackish and saline groundwater in the coastal area of the Netherlands. Neth J Geosci 82:133–147. https://doi.org/10.1017/S0016774600020692
R Core Team (2019) R: a language and environment for statistical computing Version: 3.5.3.
Schot PP, Dekker SC, Poot A (2004) The dynamic form of rainwater lenses in drained fens. J Hydrol 293:74–84. https://doi.org/10.1016/j.jhydrol.2004.01.009
Sjors H, Gunnarsson U (2002) Calcium and pH in north and central Swedish mire waters. J Ecol 90:650–657. https://doi.org/10.1046/j.1365-2745.2002.00701.x
Succow M, Jeschke L (1986) Moore in der Landschaft. Entstehung, Haushalt, Lebewelt, Verbreitung, Nutzung und Erhaltung der Moore. 2e druk. Urania Verlag, Leipzig/Jena/Berin
van der Grift B, Behrends T, Osté LA, Schot PP, Wassen MJ, Griffioen J (2016) Fe hydroxyphosphate precipitation and Fe (II) oxidation kinetics upon aeration of Fe (II) and phosphate-containing synthetic and natural solutions. Geochim Cosmochim Acta 186:71–90. https://doi.org/10.1016/j.gca.2016.04.035
van Dijk J, Stroetenga M, Bos L, van Bodegom PM, Verhoef HA, Aerts R (2004) Restoring natural seepage conditions on former agricultural grasslands does not lead to reduction of organic matter decomposition and soil nutrient dynamics. Biogeochemistry 71:317–337. https://doi.org/10.1007/s10533-004-0079-0
van Loon AH, Schot PP, Bierkens MFP, Griffioen J, Wassen MJ (2009) Local and regional impact of anthropogenic drainage on fen contiguity. Hydrol Earth Syst Sci 13:1837–1848. https://doi.org/10.5194/hess-13-1837-2009
van Wirdum G (1991) Vegetation and hydrology of floating rich-fens. PhD thesis, University of Amsterdam, Amsterdam
Wassen MJ, Barendregt A, Bootsma MC, Schot PP (1989) Groundwater chemistry and vegetation of gradients from rich fen to poor fen in the Naardermeer (the Netherlands). Vegetatio 79:117–132. https://doi.org/10.1007/BF00044904
Wassen MJ, Barendregt A, Palczynski A, de Smidt JT, de Mars H (1990) The relationship between fen vegetation radients, groundwater-flow and flooding in an undrained valley mire at Biebrza, Poland. J Ecol 78:1106–1122. https://doi.org/10.2307/2260955
Wassen MJ, Joosten JHJ (1996) In search of a hydrological explanation for vegetation changes along a fen gradient in the Biebrza Upper Basin (Poland). Vegetatio 124:191–209. https://doi.org/10.1007/BF00045494
Wassen MJ, Venterink HO, Lapshina ED, Tanneberger F (2005) Endangered plants persist under phosphorus limitation. Nature 437:547–550. https://doi.org/10.1038/nature03950
Westhoff V, Bakker PA, van Leeuwen CG, van der Voo EE, Westra R (1971) Wilde planten. Flora en vegetatie in onze natuurgebieden, deel 2: het lage land. Vereniging tot behoud van natuurmonumenten in Nederland. Amsterdam
Zak D, Gelbrecht J (2007) The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 85:141–151. https://doi.org/10.1007/s10533-007-9122-2
Acknowledgements
We would like to thank Vereniging Natuurmonumenten for allowing us access to the Naardermeer, Paul Schot for his help with fieldwork in 2018 and his comments on a draft manuscript, and Fulco Teunissen for language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marjan Jongen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Veeken, A., Wassen, M.J. Impact of local- and regional-scale restoration measures on a vulnerable rich fen in the Naardermeer nature reserve (the Netherlands). Plant Ecol 221, 893–911 (2020). https://doi.org/10.1007/s11258-020-01049-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-020-01049-6