Abstract
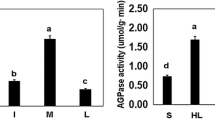
In this study, the uses of the mutated genes, upreg1 and upreg2, encoding upregulated ADP-glucose pyrophosphorylase (AGPase) large subunits with increased enzymatic activity, to improve crop yield productivity was evaluated in vitro and in planta. For in vitro examination, wild type and upregs were co-expressed with three different AGPase small subunit genes from potato and perilla to produce nine AGPase isoforms. In kinetic experiments, 3-Phosphoglycerate increased the V max and decreased the K M for the recombinant AGPase. Regardless of the specific small subunit, Upreg-type AGPases had much larger increases in enzymatic activity with concomitant decreases in values as compared to the wild type enzyme. Transformation of lettuce with the upreg1 gene altered the regulatory properties of leaf AGPase. AGPases from transgenic lettuce showed greater 3-PGA activation and lower Pi inhibition than was observed for wild type AGPase. Fresh weights of the aerial parts of transgenic plants were larger than non-transgenic controls. Based on these results, upreg mutant genes could be used for the genetic improvement of plant AGPases other than potato and effectively increase crop yield productivity.




Similar content being viewed by others
Abbreviations
- ADP-Glc:
-
ADP-glucose
- AGPase:
-
ADP-glucose pyrophosphorylase
- Glc-1-P:
-
Glucose-1-phosphate
- 3-PGA:
-
3-Phosphoglycerate
- Pi:
-
Orthophosphate
References
Ballicora MA, Laughlin MJ, Fu Y, Okita TW, Barry GF, Preiss J (1995) Adenosine 5′-diphosphate-glucose pyrophosphorylase from potato tuber. Significance of the N terminus of the small subunit for catalytic properties and heat stability. Plant Physiol 109:245–251. doi:10.1104/pp.109.1.245
Ballicora MA, Frueauf JB, Fu Y, Schűrmann P, Preiss J (2000) Activation of the potato tuber ADP-glucose pyrophosphorylase by thioredoxin. J Biol Chem 275:1315–1320. doi:10.1074/jbc.275.2.1315
Ballicora MA, Iglesias AA, Preiss J (2003) ADP-glucose pyrophosphorylase: a regulatory enzyme for bacterial glycogen synthesis. Microbiol Mol Biol Rev 67:213–225. doi:10.1128/MMBR.67.2.213-225.2003
Ballicora MA, Iglesias AA, Preiss J (2004) ADP-glucose pyrophosphorylase: a regulatory enzyme for plant starch synthesis. Photosynth Res 79:1–24. doi:10.1023/B:PRES.0000011916.67519.58
Boehlein SK, Sewell AK, Cross J, Stewart JD, Hannah LC (2005) Purification and characterization of adenosine diphosphate glucose pyrophosphorylase from maize/potato mosaics. Plant Physiol 138:1552–1562. doi:10.1104/pp.105.060699
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Carlson CA, Parsons TF, Preiss J (1976) Biosynthesis of bacterial glycogen. Activator-induced oligomerization of a mutant Escherichia coli ADP-glucose synthase. J Biol Chem 251:7886–7892
Choi SB, Kim KH, Kavakli IH, Lee SK, Okita TW (2001) Transcriptional expression characteristics and subcellular localization of ADP-glucose pyrophosphorylase in the oil plant Perilla frutescens. Plant Cell Physiol 42:146–153. doi:10.1093/pcp/pce019
Cross JM, Clancy M, Shaw JR, Greene TW, Schmidt RR, Okita TW, Hannah LC (2004) Both subunits of ADP-glucose pyrophosphorylase are regulatory. Plant Physiol 135:137–144. doi:10.1104/pp.103.036699
Cutis IS, Davey MR, Power JB (1995) Leaf disk transformation. Methods Mol Biol 44:59–70
Fu Y, Ballicora MA, Leykam JF, Preiss J (1998) Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem 273:25045–25052. doi:10.1074/jbc.273.39.25045
Ghosh HP, Preiss J (1966) Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach chloroplasts. J Biol Chem 241:4491–4504
Govons S, Vinopal R, Ingraham J, Preiss J (1969) Isolation of mutants of Escherichia coli. B altered in their ability to synthesize glycogen. J Bacteriol 97:970–972
Greene TW, Kavakli IH, Kahn ML, Okita TW (1998) Generation of up-regulated allosteric variants of potato ADP-glucose pyrophosphorylase by reversion genetics. Proc Natl Acad Sci USA 95:10322–10327. doi:10.1073/pnas.95.17.10322
Hannah LC, Giroux M, Boyer C (1993) Biotechnological modification of carbohydrates for sweet corn and maize improvement. Sci Hortic (Amsterdam) 55:177–197. doi:10.1016/0304-4238(93)90031-K
Hwang SK, Salamone PR, Okita TW (2005) Allosteric regulation of the higher plant ADP-glucose pyrophosphorylase is a product of synergy between the two subunits. FEBS Lett 579:983–990. doi:10.1016/j.febslet.2004.12.067
Iglesias AA, Barry GF, Meyer C, Blocksberg L, Nakata PA, Greene T, Laughlin MJ, Okita TG, Kishore GM, Preiss J (1993) Expression of the potato tuber ADP-glucose pyrophosphorylase in Escherichia coli. J Biol Chem 268:1081–1086
James MG, Denyer K, Myers AM (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6:215–222. doi:10.1016/S1369-5266(03)00042-6
Kavakli IH, Park JS, Slattery C, Salamone PR, Frohlick J, Okitas TW (2001a) Analysis of allosteric effector binding sites of potato ADP-glucose pyrophosphorylase through reverse genetics. J Biol Chem 276:40834–40840. doi:10.1074/jbc.M106310200
Kavakli IH, Greene TG, Salamone PR, Choi SB, Okita TW (2001b) Investigation of subunit function in ADP-glucose pyrophosphorylase. Biochem Biophys Res Commun 281:783–787. doi:10.1006/bbrc.2001.4416
Kavakli IH, Kato C, Choi SB, Kim KH, Salamone PR, Ito H, Okita TW (2002) Generation, characterization, and heterologous expression of wild-type and up-regulated forms of Arabidopsis thaliana leaf ADP-glucose pyrophosphorylase. Planta 215:430–439. doi:10.1007/s00425-001-0727-8
Li L, Preiss J (1992) Characterization of ADP-glucose pyrophosphorylase from a starch-deficient mutant of Arabidopsis thaliana (L). Carbohydr Res 227:227–239. doi:10.1016/0008-6215(92)85074-A
Meyer FD, Smidansky ED, Beecher B, Greene TW, Giroux MJ (2004) The maize Sh2r6hs ADP-glucose pyrophosphorylase (AGP) large subunit confers enhanced AGP properties in transgenic wheat (Triticum aestivum). Plant Sci 167:899–911. doi:10.1016/j.plantsci.2004.05.031
Meyer FD, Talbert LE, Martin JM, Lanning SP, Greene TW, Giroux MJ (2007) Field evaluation of transgenic wheat expressing a modified ADP-glucose pyrophosphorylase large subunit. Crop Sci 47:336–342. doi:10.2135/cropsci2006.03.0160
Obana Y, Omoto D, Kato C, Matsumoto K, Nagai Y, Kavakli IH, Hamada S, Edwards GE, Okita TW, Matsui H, Ito H (2006) Enhanced turnover of transitory starch by expression of up-regulated ADP-glucose pyrophosphorylase in Arabidopsis thaliana. Plant Sci 170:1–11. doi:10.1016/j.plantsci.2005.07.019
Okita T, Nakata PA, Anderson JM, Sowokinos JR, Morell M, Preiss J (1990) The subunit structure of potato tuber ADP-glucose pyrophosphorylase. Plant Physiol 93:785–790
Preiss J (1984) Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol 38:419–458. doi:10.1146/annurev.mi.38.100184.002223
Preiss J (1988) Biosynthesis of starch and its synthesis. In: Press J (ed) The biochemistry of plants, vol 14. Academic Press, San Diego, pp 181–254
Preiss J (1999) Biosynthesis of bacterial and mammalian glycogen and plant starch synthesis and their regulation. In: Hecht SM (ed) Bioorganic chemistry: carbohydrates. Oxford University Press, Oxford, pp 59–114
Preiss J, Sivak MN (1998) Biochemistry, molecular biology and regulation of starch synthesis. Genet Eng 20:177–223
Sakulsingharoj C, Choi SB, Hwang SK, Edwards GE, Bork J, Meyer CR, Preiss J, Okita TW (2004) Engineering starch biosynthesis for increasing rice weight: the role of the cytoplasmic ADP-glucose pyrophosphorylase. Plant Sci 167:1323–1333. doi:10.1016/j.plantsci.2004.06.028
Salamone PR, Kavakli IH, Slattery CJ, Okita TW (2002) Directed molecular evolution of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA 99:1070–1075. doi:10.1073/pnas.012603799
Slattery CJ, Kavakli IH, Okita TW (2000) Engineering starch for increased quantity and quality. Trends Plant Sci 5:291–298. doi:10.1016/S1360-1385(00)01657-5
Smidansky ED, Clancy M, Meyer FD, Lanning SP, Blake NK, Talbert LE, Giroux MJ (2002) Enhanced ADP-glucose pyrophosphorylase activity in wheat endosperm increases seed yield. Proc Natl Acad Sci USA 99:1724–1729. doi:10.1073/pnas.022635299
Smidansky ED, Martin JM, Hannah LC, Fischer AM, Giroux MJ (2003) Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 216:656–664
Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM (1992) Role of ADP-glucose pyrophosphorylase in regulating starch levels in plant tissues. Science 258:287–292. doi:10.1126/science.258.5080.287
Sweetlove LJ, Burrell MM, ap Rees T (1996a) Characterization of transgenic potato tubers with increased ADP-glucose pyrophosphorylase. Biochem J 320:487–492
Sweetlove LJ, Burrell MM, ap Rees T (1996b) Starch metabolism in tubers of transgenic potato with increased ADP-glucose pyrophosphorylase. Biochem J 320:493–498
Tiessen A, Hendriks JH, Stitt M, Branscheid A, Gibon Y, Farre EM, Geigenberger P (2002) Starch synthesis in potato tubers is upregulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to sucrose supply. Plant Cell 14:2191–2213. doi:10.1105/tpc.003640
Wang ZY, Chen XP, Wang JH, Liu TS, Liu Y, Wang GY (2007) Increasing maize seed weight by enhancing the cytoplasmic ADP-glucose pyrophosphorylase activity in transgenic maize plants. Plant Cell Tissue Organ Cult 88(1):83–92. doi:10.1007/s11240-006-9173-4
Acknowledgment
This research was supported by National Institute of agricultural Biotechnology, RDA (05-5-11-22-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SM., Ryu, TH., Kim, SI. et al. Kinetic and regulatory properties of plant ADP-glucose pyrophosphorylase genetically modified by heterologous expression of potato upreg mutants in vitro and in vivo. Plant Cell Tiss Organ Cult 96, 161–170 (2009). https://doi.org/10.1007/s11240-008-9472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9472-z