Abstract
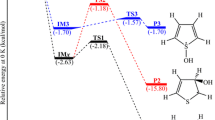
An in silico analysis of the oxidation mechanism of allyl methyl disulfide (AMDS) by hydroxyl radical was achieved at DFT level using B3LYP, CAM-B3LYP, M06-2X, and BMK functionals and 6-311++G(3df,2p) triple-zeta basis set. The calculations were carried out in both gas and aqueous phases using the SMD model (density-based solvation model). Three potential reactions were proposed according to results of Fukui function; in reactions 1 and 2, the hydroxyl radical attacks the sulfur atom breaking the disulfide bond and the reaction 3 is a hydrogen atom subtraction reaction. The respective structures of transition states (TSs) were found. Intrinsic reaction coordinate (IRC) calculations were performed for the three reactions, and their rates and equilibrium constants were calculated. When the solvent effect is taken into account, the four DFT functionals employed designate R3 (a subtraction reaction) as the fastest reaction. Thus, we elucidated the thermodynamic and kinetic feasibility of the proposed oxidation reactions.









Similar content being viewed by others
References
Casabó J, Gispert I (2007) Estructura atómica y enlace químico. Reverté, Barcelona
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:453–462
Morgan B, Van Laer K, Owusu T, Ezerina D, Pastor-Flores D, Amphonsah P, Tursch A, Dick TP (2016) Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat Chem Biol 12:437–443
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine4th edn. Clarendon Press, Oxford
Ristow M (2014) Mitohormesis explains ROS-induced health benefits. Nat Med 20:709–711
Rodriguez-Muñiz GM, Marin LM, Lhiaubet-Vallet V, Miranda MA (2012) Reactivity of nucleosides with a hydroxyl radical in non-aqueous medium. Chem Eur J 18:8024–8027
Chung LY (2016) The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food 9:205–213
Wang H, Li X, Li K, Tian J, Li J (2016) Diallyl trisulfide induces osteosarcoma cell apoptosis through reactive oxygen species-mediated downregulation of the PI3K/Akt pathway. Oncol Rep 35:3648–3658
Müller A, Eller J, Albrecht F, Prochnow P, Kuhlmann K, Bandow JE, Slusarenko AJ, Ingo L, Leichertn O (2016) Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J Biol Chem 291:11477–11490
Mahmoud YI, Riad NH, Taha H (2016) Garlic attenuates histological and histochemical alterations in livers of Schistosoma mansoni infected mice. Biotech Histochem 91:389–395
Chandra-Kuntal K, Lee J, Singh SV (2013) Critical role for reactive oxygen species in apoptosis inductionand cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res Treat 138:69–79
Choi YH, Park HS (2012) Apoptosis induction of U937 human leukemiacells by diallyl trisulfide induces through generation of reactive oxygen species. J Biomed Sci 19:50
Kaschula CH, Hunter R, Hassan HT, Stellenboom N, Cotton J, Zhai XQ, Parker MI (2011) Anti-proliferative activity of synthetic ajoene analogues on cancer cell-lines. Anti Cancer Agents Med Chem 11:260–266
Wang X, Liu R, Yang Y, Zhang M (2015) Isolation, purification and identification of antioxidants in an aqueous aged garlic extract. Food Chem 187:37–43
Li FM, Li T, Li W, Yang LD (2015) Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind Crop Prod 69:137–142
Baptista L, da Silva EC, Arbilla G (2008) Oxidation mechanism of dimethyl sulfoxide (DMSO) by OH radical in liquid phase. Phys Chem Chem Phys 10:6867–6879
Bil A, Grzechnik K, Mierzwicki K, Mielke Z (2013) OH-induced oxidative cleavage of dimethyl disulfide in the presence of NO. J Phys Chem A 117:8263–8273
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5653
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Raghavachari K, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. 20. Basis set for correlated wave-functions. J Chem Phys 72:650–654
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106:4049–4050
Davila YA, Sancho MI, Almandoz MC, Gasull E (2018) Spectroscopic and electronic analysis of chelation reactions of galangin and related flavonoids with nickel(II). J Chem Eng Data 63:1488–1497
Yanai T, Tew D, Handy N (2004) A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Boese AD, Martin JML (2004) Development of density functionals for thermochemical kinetics. J Chem Phys 121:3405–3416
Peng C, Schlegel HB (1993) Combining synchronous transit and quasi-Newton methods for finding transition states. Israel J Chem 33:449–454
Li H, Zhang Y, Wan J, Xiao H, Chen X (2018) Theoretical investigation on the oxidation mechanism of dibutyl phthalate by hydroxyl and sulfate radicals in the gas and aqueous phase. Chem Eng J 339:381–392
Ma F, Xie HB, Chen J (2017) Benchmarking of DFT functionals for the kinetics and mechanisms of atmospheric addition reactions of OH radicals with phenyl and substituted phenyl-based organic pollutants. Int J Quantum Chem. https://doi.org/10.1002/qua.25533
Espinoza S, Lezama J, Mora JR, Cordova T, Chuchani G (2016) Theoretical calculations on the mechanism of the elimination kinetics of allyl cyclohexyl-, -amine, -sulfide, -ether, and allyl ethyl ether in thegas phase. Comput Theor Chem 1090:6–16
Galano A, Alvarez-Idaboy JR (2014) Kinetics of radical-molecule reactions in aqueous solution: a benchmark study of the performance of density functional methods. J Comput Chem 35:2019–2026
Martinez-De HJM, Konstantinov IA, Arturo SG, Dombrowski G (2016) Theoretical study of reactions between oxygen-centered radicals (·OH and SO4 ·−) and vinyl monomers in aqueous phase. Macromol Theory Simul 25:475–481
Karton A, Tarnopolsky A, Lamere JF, Schatz GC, Martin JML (2008) Highly accurate first-principles benchmark data sets for the parametrization and validation of density functional and other approximate methods. Derivation of a robust, generally applicable, double-hybrid functional for thermochemistry and thermochemical kinetics. J Phys Chem A 112:12868–12886
Zheng G, Zhao Y, Truhlar DG (2007) Thermochemical kinetics of hydrogen atom transfers between methyl, methane, ethynyl, ethyne, and hydrogen. J Phys Chem A 111:4632–4642
Ponnusamy S, Sandhiya L, Senthilkumar K (2017) The atmospheric oxidation mechanism and kinetics of 1,3,5-trimethylbenzene initiated by OH radicals—a theoretical study. New J Chem 41:10259–10271
Ponnusamy S, Sandhiya L, Senthilkumar K (2017) Mechanism and kinetics of the reaction of nitrosamines with OH radical: a theoretical study. In J Chem Kinet 49:339–353
Eyring H (1935) The activated complex in chemical reactions. J Chem Phys 3:107–115
Sun H, Law CK (2010) Kinetics of hydrogen abstraction reactions of butene isomers by OH radical. J Phys Chem A 114:12088–12098
Uranga J, Mujika JI, Matxain JM (2015) •OH oxidation toward S- and OH-containing amino acids. J Phys Chem B 119:15430–15442
Acknowledgments
Mario G. Díaz gratefully acknowledges a doctoral fellowship from CONICET.
Funding
This study is financially supported by the UNSL and CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Díaz, M.G., Andrada, M.F., Vega-Hissi, E.G. et al. Density functional theory study of the oxidation reaction in the gas and aqueous phase of allyl methyl disulfide with hydroxyl radical. Struct Chem 30, 237–245 (2019). https://doi.org/10.1007/s11224-018-1198-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1198-x