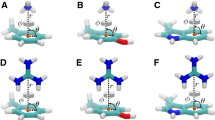
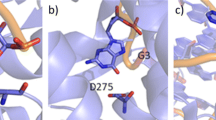
Abstract
Cation–π interactions are functionally relevant, strong secondary interactions that play versatile roles in a variety of chemical and biological systems. Therefore, it is very important to be able to describe accurately and reliably these interactions. In this study, we propose a methodology for the accurate modeling of cation–π interactions in proteins using QM/MM calculations. We developed a methodology for computing the many-body interaction energy terms and tested the effect of various factors on the accuracy of the binding energy. We found that once well-equilibrated structures were reached in the MD simulations, very similar results can be obtained for the various snapshots taken from the trajectory. The calculated interaction energies were only slightly influenced by electrostatic embedding of the point charges in the QM/MM calculations and by QM/MM geometry optimization. The calculated molecular mechanics interaction energies were off by 50 % for cation–π interactions. Instead, we suggest the calibration of force fields based on fragment-based QM calculations on geometries obtained from MD simulations to yield reliable binding energies at reduced computational cost.






Similar content being viewed by others
References
Cerny J, Hobza P (2007) Non-covalent interactions in biomacromolecules. Phys Chem Chem Phys 9:5291–5303
Cheng T, Li Q, Zhou Z, Wang Y, Bryant SH (2012) Structure-based virtual screening for drug discovery: a problem-centric review. AAPS J 14:133–141
Zacharias N, Dougherty DA (2002) Cation-pi interactions in ligand recognition and catalysis. Trends Pharmacol Sci 23:281–287
Dougherty DA (2013) The cation-π interaction. Acc Chem Res 46:885–893
Luhmer M, Bartik K, Dejaegere A, Bovy P, Reisse J (1994) The importance of quadrupolar interactions in molecular recognition processes involving a phenyl group. Bull Soc Chim Fr 131:603–606
Williams JH (1993) The molecular electric quadrupole-moment and solid-state architecture. Acc Chem Res 26:593–598
McCurdy A, Jimenez L, Stauffer DA, Dougherty DA (1992) Biomimetic catalysis of Sn2 reactions through cation-pi interactions—the role of polarizability in catalysis. J Am Chem Soc 114:10314–10321
Ma JC, Dougherty DA (1997) The cation-pi interaction. Chem Rev 97:1303–1324
Gallivan JP, Dougherty DA (1999) Cation-pi interactions in structural biology. Proc Natl Acad Sci USA 96:9459–9464
Martz E (2002) Protein explorer: easy yet powerful macromolecular visualization. Trends Biochem Sci 27:107–109
Scior T, Bender A, Tresadern G, Medina-Franco JL, Martinez-Mayorga K, Langer T, Cuanalo-Contreras K, Agrafiotis DK (2012) Recognizing pitfalls in virtual screening: a critical review. J Chem Inf Model 52:867–881
Wein S, Maynadier M, Bordat Y, Perez J, Maheshwari S, Bette-Bobillo P, Ba CTV, Penarete-Vargas D, Fraisse L, Cerdan R, Vial H (2012) Transport and pharmacodynamics of albitiazolium, an antimalarial drug candidate. Br J Pharmacol 166:2263–2276
Vial HJ, Wein S, Farenc C, Kocken C, Nicolas O, Ancelin ML, Bressolle F, Thomas A, Calas M (2004) Prodrugs of bisthiazolium salts are orally potent antimalarials. Proc Natl Acad Sci USA 101:15458–15463
Nagy GN, Marton L, Contet A, Ozohanics O, Ardelean L-M, Revesz A, Vekey K, Irimie FD, Vial H, Cerdan R, Vertessy BG (2014) Composite aromatic boxes for enzymatic transformations of quaternary ammonium substrates. Angew Chem Int Ed 53:13471–13476
Kwak BY, Zhang YM, Yun M, Heath RJ, Rock CO, Jackowski S, Park HW (2002) Structure and mechanism of ctp: phosphocholine cytidylyltransferase (licc) from Streptococcus pneumoniae. J Biol Chem 277:4343–4350
Veitch DP, Gilham D, Cornell RB (1998) The role of histidine residues in the hxgh site of ctp: phosphocholine cytidylyltransferase in ctp binding and catalysis. Eur J Biochem 255:227–234
Lee J, Johnson J, Ding Z, Paetzel M, Cornell RB (2009) Crystal structure of a mammalian ctp: phosphocholine cytidylyltransferase catalytic domain reveals novel active site residues within a highly conserved nucleotidyltransferase fold. J Biol Chem 284:33535–33548
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Zhao Y, Truhlar DG (2008) The m06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four m06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Becke AD (1993) Density-functional thermochemistry iii. The role of exact exchange. J Chem Phys 98:5648–5652
Tao JM, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements H-Pu. J Chem Phys 132:154104
Kendall RA, Dunning TH, Harrison RJ (1992) Electron-affinities of the 1st-row atoms revisited—systematic basis-sets and wave-functions. J Chem Phys 96:6796–6806
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09. Revision A.1 edn. Gaussian, Inc., Wallingford, CT
Boys SF, Bernardi F (2002) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors (reprinted from molecular physics, vol 19, p 553–566, 1970). Mol Phys 100:65–73
Glendening ED, Streitwieser A (1994) Natural energy decomposition analysis—an energy partitioning procedure for molecular-interactions with application to weak hydrogen-bonding, strong ionic, and moderate donor–acceptor interactions. J Chem Phys 100:2900–2909
Weinhold F (2012) Natural bond orbital analysis: a critical overview of relationships to alternative bonding perspectives. J Comput Chem 33:2363–2379
Nagy GN, Marton L, Krámos B, Oláh J, Révész Á, Vékey K, Delsuc F, Hunyadi-Gulyás É, Medzihradszky KF, Lavigne M, Vial H, Cerdan R, Vértessy BG (2013) Evolutionary and mechanistic insights into substrate and product accommodation of ctp: phosphocholine cytidylyltransferase from Plasmodium falciparum. FEBS J 280:3132–3148
Li H, Robertson AD, Jensen JH (2005) Very fast empirical prediction and rationalization of protein pk(a) values. Proteins: Struct, Funct, Bioinf 61:704–721
Bas DC, Rogers DM, Jensen JH (2008) Very fast prediction and rationalization of pk(a) values for protein-ligand complexes. Proteins Struct Funct Bioinform 73:765–783
Olsson MHM, Sondergaard CR, Rostkowski M, Jensen JH (2011) Propka3: consistent treatment of internal and surface residues in empirical pk(a) predictions. J Chem Theory Comput 7:525–537
Sondergaard CR, Olsson MHM, Rostkowski M, Jensen JH (2011) Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pk(a) values. J Chem Theory Comput 7:2284–2295
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Brooks BR, Brooks CL III, Mackerell AD Jr, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, Caflisch A, Caves L, Cui Q, Dinner AR, Feig M, Fischer S, Gao J, Hodoscek M, Im W, Kuczera K, Lazaridis T, Ma J, Ovchinnikov V, Paci E, Pastor RW, Post CB, Pu JZ, Schaefer M, Tidor B, Venable RM, Woodcock HL, Wu X, Yang W, York DM, Karplus M (2009) Charmm: the biomolecular simulation program. J Comput Chem 30:1545–1614
MacKerell AD, Banavali N, Foloppe N (2000) Development and current status of the CHARMM force field for nucleic acids. Biopolymers 56:257–265
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph Model 14:33–38
Feller SE, MacKerell AD (2000) An improved empirical potential energy function for molecular simulations of phospholipids. J Phys Chem B 104(31):7510–7515
Harvey JN (2004) Spin-forbidden co ligand recombination in myoglobin. Faraday Discuss 127:165–177
Tinker—home page. Tinker—software tools for molecular design. http://dasher.Wustl.Edu/tinker/. Accessed 5 Oct 2011
Ren P, Wu C, Ponder JW (2011) Polarizable atomic multipole-based molecular mechanics for organic molecules. J Chem Theory Comput 7:3143–3161
Hodges MP, Stone AJ, Xantheas SS (1997) Contribution of many-body terms to the energy for small water clusters: a comparison of ab initio calculations and accurate model potentials. J Phys Chem A 101:9163–9168
Xantheas SS (2000) Cooperativity and hydrogen bonding network in water clusters. Chem Phys 258:225–231
Padgett CL, Hanek AP, Lester HA, Dougherty DA, Lummis SC (2007) Unnatural amino acid mutagenesis of the gaba(a) receptor binding site residues reveals a novel cation-pi interaction between gaba and beta 2tyr97. J Neurosci 27:886–892
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2009) NBO 5.9. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI
Olah J, van Bergen L, De Proft F, Roos G (2015) How does the protein environment optimize the thermodynamics of thiol sulfenylation? Insights from model systems to qm/mm calculations on human 2-cys peroxiredoxin. J Biomol Struct Dyn 33:584–596
Lonsdale R, Olah J, Mulholland AJ, Harvey JN (2011) Does compound I vary significantly between isoforms of cytochrome p450? J Am Chem Soc 133:15464–15474
Saha S, Sastry GN (2015) Cooperative or anticooperative: how noncovalent interactions influence each other. J Phys Chem B ASAP. doi:10.1021/acs.jpcb.5b03005
Xu M, Lill MA (2013) Induced fit docking, and the use of qm/mm methods in docking. Drug Discov Today Technol 10:e411–e418
Yilmazer ND, Korth M (2013) Comparison of molecular mechanics, semi-empirical quantum mechanical, and density functional theory methods for scoring protein–ligand interactions. J Phys Chem B 117:8075–8084
Acknowledgments
The authors thank Dr. Goedele Roos (ULB, Belgium), Gergely N. Nagy, and Dr. Andras T. Rokob (MTA TTK, Hungary) for careful reading of the manuscript and helpful discussions. We are grateful for the support of the New Széchenyi Plan TAMOP-4.2.2/B-10/1-2010-0009 and for the financial support of OTKA Grant No. 108721. J.O. acknowledges receipt of a Bolyai János Research Fellowship. A.L. acknowledges the financial support of Richter Gedeon Talentum Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Magdolna Hargittai on the occasion of her 70th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lábas, A., Krámos, B., Bakó, I. et al. Accurate modeling of cation–π interactions in enzymes: a case study on the CDPCho:phosphocholine cytidylyltransferase complex. Struct Chem 26, 1411–1423 (2015). https://doi.org/10.1007/s11224-015-0658-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0658-9