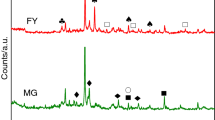
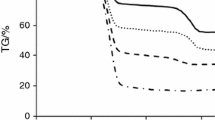
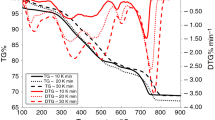
Abstract
The present work provides a detailed characterization and kinetic study of oil shale of Upper Assam, India. The physicochemical characteristics of oil shale were studied by proximate analysis, elemental analysis, Fourier transform infrared spectroscopy and X-ray diffraction. The physicochemical study showed the oil shale to be of siliceous type, sour in the presence of aliphatic, aromatic and phenolic compounds. The thermal decomposition of the oil shale was studied using thermogravimetric analysis at heating rates of 10, 20, 30 and 50 °C min−1. The kinetic study of oil shale pyrolysis process was performed on the thermogravimetric data using three model-free isoconversional methods, viz. Friedman, Flynn–Wall–Ozawa and Kissinger–Akahira–Sunose. The reaction mechanisms were determined using the Criado master plot. The understanding of the composition of Indian oil shale and pyrolysis process kinetics can help establishing the experimental parameters for the extraction of valuable products from the oil shale.








Similar content being viewed by others
Abbreviations
- AC:
-
Ash content
- DTG:
-
Differential thermogravimetry
- FC:
-
Fixed carbon
- FTIR:
-
Fourier transform infrared spectroscopy
- FWO:
-
Flynn–Wall–Ozawa
- KAS:
-
Kissinger–Akahira–Sunose
- MC:
-
Moisture content
- TOC:
-
Total organic carbon
- TG:
-
Thermogravimetric analysis
- VM:
-
Volatile matter
- XRD:
-
X-ray diffraction
- A :
-
Pre-exponential factor (s−1)
- C:
-
Carbon
- E :
-
Activation energy (kJ mol−1)
- f(α):
-
Reaction mechanism
- g(α):
-
Integral form of reaction mechanism
- H:
-
Hydrogen
- k(T) or k :
-
Reaction rate constant (s−1)
- N:
-
Nitrogen
- R :
-
Gas constant (8.314 J mol−1 K−1)
- S:
-
Sulfur
- T :
-
Absolute temperature (K)
- w o :
-
Initial mass
- w T :
-
Mass at temperature, T
- w α :
-
Mass at conversion, α
- t :
-
Conversion time (s)
- α :
-
Conversion degree
- β :
-
Heating rate (°C min−1)
References
Gwyn JE. Oil from shale as a viable replacement of depleted crude reserves: processes and challenges. Fuel Process Technol. 2001;70:27–40.
Tiwari P. Oil shale pyrolysis: benchscale experimental studies and modelling. Utah: University of Utah; 2012.
Braun RL, Rothman AJ. Oil shale pyrolysis: kinetics and mechanism of oil production. Fuel. 1975;54(April):129–31.
Charlesworth JM. Oil shale pyrolysis: 1. Time and temperature dependance of product composition. Ind Eng Chem Process Des Dev. 1985;24:1117–25.
Solomon PR, Carengelo RM, Horn E. The effect of pyrolysis conditions on Israeli oil shale particles. Fuel. 1986;65(May):650–62.
Li S, Qian J. Study of the pyrolysis of Maoming oil shale lumps. Fuel. 1991;70:1371–5.
Stirzhakova YA, Usova TV. Current trends in pyrolysis of oil shale: a review. Solid Fuel Chem. 2008;42(4):197–201.
Ren L, Xia D, Xu Y, Guo M, Sun H, Liu X. Research on pyrolysis mechanism of Huadian oil shale. Energy Procedia. 2015;66:13–6. https://doi.org/10.1016/j.egypro.2015.02.007.
Nazzal JM. Influence of heating rate on the pyrolysis of Jordanian oil shale. J Anal Appl Pyrol. 2002;62:225–38.
Han X, Liu Q, Jiang X. Heat transfer characteristics of oil shale particle during the retorting. Int J Heat Mass Transf. 2015;84:578–83.
Kumar R, Bansal V, Badhe RM, Madhira ISS, Sugumaran V, Ahmed S, et al. Characterization of Indian origin oil shale using advanced analytical techniques. Fuel. 2013;113:610–6. https://doi.org/10.1016/j.fuel.2013.05.055.
Hutton AC. Petrographic classification of oil shale. Int J Coal Geol. 1987;8:203–31.
Cook AC, Sherwood NR. Clasification of oil shales, coal and other organic rocks. Org Geochem. 1991;17(2):211–22.
Patterson JH. A review on the effects of minerals in processing of Australian oil shales. Fuel. 1994;73(3):321–7.
Patterson JH, Hurst HJ, Levy JH, Killingley JS. Mineral reactions in the processing of Australian Tertiary oil shales. Fuel. 1990;69(September):1119–23.
Patterson JH, Hurst HJ, Levy JH. Relevance of carbonate minerals in the processing of Australian Tertiary oil shales. Fuel. 1991;70(November):1252–9.
Tiwari P, Deo M. Detailed kinetic analysis of oil shale using TGA data. AIChE. 2012;58(2):505–16. https://doi.org/10.1002/aic.12589.
Bai F, Sun Y, Liu Y, Li Q, Guo M. Thermal and kinetic characteristics of pyrolysis and combustion of three oil shales. Energy Convers Manag. 2015;97:374–81.
Jaber JO, Probert SD. Non-isothermal thermogravimetry and decomposition kinetics of two Jordanian oil shales under different processing conditions. Fuel Process Technol. 2000;63:57–70.
Ekstrom A, Callaghan G. The pyrolysis kinetics of some Australian oil shales. Fuel. 1987;66(March):331–7.
Skala D, Kopsch H, Sokic M, Neumann HJ, Jovanovic J. Modelling and simulation of oil shale pyrolysis. Fuel. 1989;68(February):168–73.
Han H, Nn Zhong, Cx Huang, Zhang W. Pyrolysis kinetics of oil shale from northeast China: implications from thermogravimetric and Rock-Eval experiments. Fuel. 2015;157:776–83. https://doi.org/10.1016/j.fuel.2015.07.052.
Braun RL, Burnham AK. Kinetics of Colorado oil shale pyrolysis in a fluidized-bed reactor. Fuel. 1986;65:218–22.
Aboulkas A, Harfi KE. Study of the kinetics and mechanisms of thermal decomposition of Moroccan Tarfaya oil shale and its kerogen. Oil Shales. 2008;25(4):426–43. https://doi.org/10.3176/oil.2008.4.04.
Harahsheh MA, Ayed OA, Robinson J, Kingman S, Harahsheh AA, Tarawneh K, et al. Effect of demineralization and heating rate on the pyrolysis kinetics of Jordanian oil shales. Fuel Process Technol. 2011;92:1805–11. https://doi.org/10.1016/j.fuproc.2011.04.037.
Vyazovkin S, Wight CA. Model free and model fitting approaches to kinetic analysis of isothermal and non-isothermal data. Thermochim Acta. 1999;340–341:53–68.
Youhong S, Shuai Z, Qiang L, Shichang L, Jing H. Thermoelectric coupling analysis of high-voltage breakdown industrial frequency pyrolysis in Fuyu oil shale. Int J Therm Sci. 2018;130:19–27. https://doi.org/10.1016/j.ijthermalsci.2018.03.013.
Sańchez-Jimeńez PE, Perez-Maqueda LA, Perejón A, Criado JM. Generalized kinetic master plots for the thermal degradation of polymers following a random scission mechanism. J Phys Chem A. 2010;114:7868–76.
Gelman F, Binstock R, Halicz L. Application of the Walkley–Black titration for the organic carbon quantification in organic rich sedimentary rocks. Fuel. 2012;96:608–10. https://doi.org/10.1016/j.fuel.2011.12.053.
Torrente MC, Galan MA. Kinetics of the thermal decomposition of oil shale from Puertollano (Spain). Fuel. 2001;80:327–34.
Li S, Yue C. Study of different kinetic models for oil shale pyrolysis. Fuel Process Technol. 2003;85:51–61. https://doi.org/10.1016/S0378-3820(03)00097-3.
Vyazovkin S. Model free kinetics, staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83(1):45–51.
Vyazovkin S, Burnham AK, Criado JM, Parez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetic committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Al-makhadmeh LA, Maier J, Batiha MA, Scheffknecht G. Oxyfuel technology: oil shale desulfurization behavior during staged combustion. Fuel. 2017;190:229–36. https://doi.org/10.1016/j.fuel.2016.11.022.
Yu X, Luo Z, Li H, Gan D. Effect of vibration on the separation efficiency of oil shale in a compound dry separator. Fuel. 2018;214:242–53. https://doi.org/10.1016/j.fuel.2017.10.129.
Maes J, Muggeridge AH, Jackson MD, Quintard M, Lapene A. Scaling analysis of the In-Situ upgrading of heavy oil and oil shale. Fuel. 2017;195:299–313. https://doi.org/10.1016/j.fuel.2017.01.072.
Wang Q, Hou Y, Wu W, Liu Q, Liu Z. The structural characteristics of kerogens in oil shale with different density grades. Fuel. 2018;219:151–8. https://doi.org/10.1016/j.fuel.2018.01.079.
Gotor FJ, Criado JM, Malek J, Koga N. Kinetic analysis of solid-state reactions: the universality of master plots for analyzing isothermal and nonisothermal experiments. J Phys Chem. 2000;104:10777–82.
Criado JM. Kinetic analysis of DTG data from master curve. Thermochim Acta. 1978;24:186–9.
Criado JM, Malek J, Ortega A. Applicability of the master plots in kinetic analysis of non-isothermal data. Thermochim Acta. 1989;147:377–85.
Sánchez-Jiménez PE, Pérez-Maqueda LA, Perejón A, Criado JM. Generalized master plots as a straightforward approach for determining the kinetic model: the case of cellulose pyrolysis. Thermochim Acta. 2013;552:54–9. https://doi.org/10.1016/j.tca.2012.11.003.
Sánchez-Jiménez PE, Pérez-Maqueda LA, Perejón A, Criado JM. A new model for the kinetic analysis of thermal degradation of polymers driven by random scission. Polym Degrad Stab. 2010;95(5):733–9. https://doi.org/10.1016/j.polymdegradstab.2010.02.017.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally simulated reactions of solid. Int Rev Phys Chem. 1998;17(3):407–33.
Bai F, Guo W, Lu X, Liu Y, Guo M, Li Q, et al. Kinetic study on the pyrolysis behaviour of Huadian oil shale via non isothermal thermogravimetric data. Fuel. 2015;146:111–8. https://doi.org/10.1016/j.fuel.2014.12.073.
Loo L, Maaten B, Siirde A, Pihu T, Konist A. Experimental analysis of the combustion characteristics of Estonian oil shale in air and oxy-fuel atmospheres. Fuel Process Technol. 2015;134:317–24. https://doi.org/10.1016/j.fuproc.2014.12.051.
Tiwari P, Deo M. Compositional and kinetic analysis of oil shale pyrolysis using TGA-MS. Fuel. 2012;94:333–41. https://doi.org/10.1016/j.fuel.2011.09.018.
Amer MW, Marshall M, Fei Y, Jackson WR, Gorbaty ML, Cassidy PJ, et al. The structure and reactivity of a low-sulfur lacustrine oil shale (Colorado U.S.A.) compared with those of a high-sulfur marine oil shale (Julia Creek, Queensland, Australia). Fuel Process Technol. 2015;135:91–8. https://doi.org/10.1016/j.fuproc.2014.10.032.
Wang Z, Deng S, Gu Q, Zhang Y, Cui X, Wang H. Pyrolysis kinetic study of Huadian oil shale, spent oil shale and their mixtures by thermogravimetric analysis. Fuel Process Technol. 2013;110:103–8. https://doi.org/10.1016/j.fuproc.2012.12.001.
Ec Moine, Groune K, El Hamidi A, Khachani M, Halim M, Arsalane S. Multistep process kinetics of the non-isothermal pyrolysis of Moroccan Rif oil shale. Energy. 2016;115:931–41. https://doi.org/10.1016/j.energy.2016.09.033.
El Nady MM, Hammad MM. Organic richness, kerogen types and maturity in the shales of the Dakhla and Duwi formations in Abu Tartur area, Western Desert, Egypt: implication of Rock-Eval pyrolysis. Egypt J Pet. 2015;24(4):423–8. https://doi.org/10.1016/j.ejpe.2015.10.003.
Gerasimov G, Khaskhachikh V, Potapov O. Experimental study of kukersite oil shale pyrolysis by solid heat carrier. Fuel Process Technol. 2017;158:123–9. https://doi.org/10.1016/j.fuproc.2016.12.016.
Lewan MD, Roy S. Role of water in hydrocarbon generation from Type-I kerogen in Mahogany oil shale of the Green River Formation. Org Geochem. 2011;42(1):31–41. https://doi.org/10.1016/j.orggeochem.2010.10.004.
Maaten B, Loo L, Konist A, Pihu T, Siirde A. Investigation of the evolution of sulphur during the thermal degradation of different oil shales. J Anal Appl Pyrolysis. 2017;128:405–11. https://doi.org/10.1016/j.jaap.2017.09.007.
Gregg ML, Campbell JH, Taylor JR. Laboratory and modelling investigation of a Colorado oil shale block heated at 900°C. Fuel. 1981;60(March):179–88.
Xu J, Meng Q, Li B, Liu R, Xu Y, Gratzer R, et al. Oil yield and bulk geochemical parameters of oil shales from the Songliao and Huadian Basins, China: a grade classification approach. Oil Shale. 2013;30(3):402. https://doi.org/10.3176/oil.2013.3.03.
Acknowledgements
The authors would like to offer sincere thanks to Coal India Limited, NEC Coal Field, Margherita, Assam, for providing the samples and Assam Rifles for providing access into the coal mines for collecting the samples. Authors are immensely thankful to Sophisticated and Analytical Instruments Centre (SAIC) at Tezpur University and Department of Chemical Engineering at IIT Guwahati for providing various analytical facilities.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baruah, B., Kataki, R., Thakur, P. et al. Detailed physicochemical and thermochemical investigation of Upper Assam oil shale. J Therm Anal Calorim 138, 1221–1232 (2019). https://doi.org/10.1007/s10973-019-08163-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08163-2