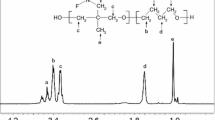
Abstract
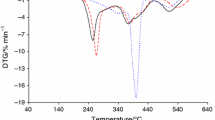
In this paper, the thermal degradation properties of Viton A and Fluorel are investigated by both isoconversional and combined kinetic analysis methods using non-isothermal thermogravimetry technique. It has been found that the heating rate has little affect on the degradation residue of Fluorel and Viton A, where around 1.3% char was formed for Fluorel and 3.5% for Viton A. Different from the literature, the decomposition of Viton A should be considered as an overlapped dehydrofluorination and carbon chain scission process, with activation energy of 214 ± 11 and 268 ± 13 kJ mol−1, respectively. The effect of dehydrofluorination on degradation of Fluorel is not so significant due to low content of H, and hence, it could be considered as a single-step mechanism with average activation energy of 264 ± 14 kJ mol−1. The thermal stability of Fluorel is much better than that of Viton A, and the predicted half-life is around 218 min for Fluorel and 49 min for Viton A at 420 °C, which are consistent with experimental values. If using a single-step model as in the literature for Viton A, its half-life at 420 °C would be underestimated for >20%.









Similar content being viewed by others
References
Nouguez B, Mahé B, Vignaud PO. Cast PBX related technologies for IM shells and warheads. Sci Tech Energ Mater. 2009;70(5–6):135–9.
Yarrington CD, Son SF, Foley TJ. Combustion of silicon/teflon/viton and aluminum/teflon/viton energetic composites. J Propuls Power. 2010;26(4):734–43.
Hoffman DM. DMA signatures of Viton A and plastic bonded explosives based on this polymer. Polym Eng Sci. 2003;43(1):139–56.
Yan Q-L, Zeman S, Elbeih A. Recent advances in thermal analysis and stability evaluation of insensitive plastic bonded explosives (PBXs). Thermochim Acta. 2012;537:1–12.
Sossi A, Duranti E, Paravan C, Deluca LT, Vorozhtsov AB, Gromov AA, Pautov YI, Lerner MI, Rodkevich NG. Non-isothermal oxidation of aluminum nanopowder coated by hydrocarbons and fluorohydrocarbons. Appl Surf Sci. 2013;271(15):337–43.
Lee J-S. Thermal decomposition of fluoropolymers and firing characteristics of priming compositions with fluoropolymers. J Appl Polym Sci. 2005;97(5):2054–9.
Hoffman DM. DMA signatures of Viton A and plastic bonded explosives based on this polymer. Polym Eng Sci. 2003;43(1):139–56.
Singh G, Felix SP, Soni P. Studies on energetic compounds Part 31: thermolysis and kinetics of RDX and some of its plastic bonded explosives. Thermochim Acta. 2005;426:131–9.
Felix SP, Singh G, Sikder AK, Aggrawal JP. Studies on energetic compounds-Part 33: thermolysis of keto-RDX and its plastic bonded explosives containing thermally stable polymers. Thermochim Acta. 2005;426:53–60.
Long GT, Vyazovkin S, Brems BA, Wight CA. Competitive vaporization and decomposition of liquid RDX. J Phys Chem B. 2000;104:2570–4.
Zeman S, Elbeih A, Akstein Z. Preliminary study on several plastic bonded explosives based on cyclic nitramines. Chin J Energ Mater. 2011;19(1):8–12.
Elbeih A, Pachmán J, Zeman S, Trzcinski AW, Akstein Z, Muhamed S. Thermal stability and detonation characteristics of pressed and elastic explosives on the basis of selected cyclic nitramines. Cent Eur J Energ Mater. 2010;7(3):217–32.
Wemhoff AP, Howard WM, Burnham AK, Nichols AL III. An LX-10 kinetic model calibrated using simulations of multiple small-scale thermal safety tests. J Phys Chem A. 2008;112(38):9005–11.
Yan Q-L, Zeman S, Elbeih A. Thermal behavior and decomposition kinetics of Viton A bonded explosives containing attractive cyclic nitramines. Thermochim Acta. 2013;562(20):56–64.
Yan Q-L, Zeman S, Zhang T-L, Elbeih A. Non-isothermal decomposition behavior of Fluorel bonded explosives containing attractive cyclic nitramines. Thermochim Acta. 2013;574:10–8.
Knight GJ, Wright WW. The thermal degradation of hydrofluoro polymers. J Appl Polym Sci. 1972;16:683–93.
Cuccuru A, Sodi F. Thermal stability in air of gamma irradiated fluoroelastomer. Thermochim Acta. 1976;15:253–6.
Papazian HA. Prediction of polymer degradation kinetics at moderate temperatures from TGA Measurements. J Appl Polym Sci. 1972;16:2503–10.
Burnham AK, Weese RK. Kinetics of thermal degradation of explosive binders Viton A, Estane, and Kel-F. Thermochim Acta. 2005;426:85–92.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Sánchez-Jiménez PE. Comments on “thermal decomposition of pyridoxine: an evolved gas analysis-ion attachment mass spectrometry study”. About the application of model-fitting methods of kinetic analysis to single non-isothermal curves. Rapid Commun Mass Spectrom. 2013;27:500–2.
Sanchez-Jimenez PE, Perez-Maqueda LA, Perejon A, Criado JM. Constant rate thermal analysis for thermal stability studies of polymers. Polym Degrad Stab. 2011;96(5):974–81.
David C. In: Bamford CH, Tipper CFH, editors. EBST comprehensive chemical kinetics, vol. 14. Amsterdam: Elsevier; 1977. p. 1–173.
Wright WW. In: Robb JC, Peaker FW, editors. Progress in high polymers, vol. 2. London: Heywood; 1968. p. 193.
Degteva TG, Sedova IM, Kuzminskii AS. Thermal degradation of a fluorinated elastomer of the Kel-F type at temperatures above 3001C-II. Polym Sci USSR. 1964;5:582.
Madorsky SL. Thermal degradation of organic polymers. New York: Interscience; 1964.
Perez-Maqueda LA, Criado JM, Sanchez-Jimenez PE. Combined kinetic analysis of solid-state reactions: a powerful tool for the simultaneous determination of kinetic parameters and the kinetic model without previous assumptions on the reaction mechanism. J Phys Chem A. 2006;110(45):12456–62.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109(3):1203–14.
Yan Q-L, Zeman S, Svoboda R, Elbeih A, Málek J. The effect of crystal structure on the thermal initiation of CL-20 and its C4 bonded explosives (II): models for overlapped reactions and thermal stability. J Therm Anal Calorim. 2013;112(2):837–49.
Svoboda R, Málek J. Applicability of Fraser-Suzuki function in kinetic analysis of complex crystallization processes. J Therm Anal Calorim. 2013;111(2):1–12.
Perejón A, Sánchez-Jiménez PE, Criado JM, Pérez-Maqueda LA. Kinetic analysis of complex solid-state reactions. A new deconvolution procedure. J Phys Chem B. 2011;115(8):1780–91.
Wall LA. Fluoropolymers. New York: Wiley Interscience; 1972.
Banik I, Bhowmick AK, Raghavan SV, Majali AB, Tikku VK. Thermal degradation studies of electron beam cured terpolymeric Fluorocarbon rubber. Polym Degrad Stab. 1999;63:413–21.
Dutta SK, Bhowmick AK, Mukunda PG, Chaki TK. Thermal degradation studies of electron beam cured ethylene-vinyl acetate copolymer. Polym Degrad Stab. 1995;50(1):75–82.
Sanchez-Jimenez PE, Perez-Maqueda LA, Perejon A, Criado JM. A new model for the kinetic analysis of thermal degradation of polymers driven by random scission. Polym Degrad Stab. 2010;95(5):733–9.
Burnham AK, Braun RL, Coburn TT, Sandvik EI, Curry DJ, Schmidt BJ, Noble RA. An appropriate kinetic model for well-preserved algal kerogens. Energ Fuels. 1996;10:49–59.
Nichols AL III. Improving the model fidelity for the mechanical response in a thermal cookoff of HMX. AIP Conf Proc. 2012;1426:551–4.
Acknowledgements
The authors would like to present special thanks to ERASMUS (2013–2014) program for the financial support on cooperation between University of Pardubice in Czech Republic and University of Sevilla in Spain. Valuable discussion on kinetic prediction with Dr. P. E. Sánchez Jiménez from Instituto de Ciencia de Materiales de Sevilla, CSIC-Universidad de Sevilla is also greatly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, SE., Zhou, WL., Yan, QL. et al. New findings on thermal degradation properties of fluoropolymers. J Therm Anal Calorim 128, 675–685 (2017). https://doi.org/10.1007/s10973-016-5963-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5963-z