Abstract
The present study was conducted to determine the oxidative stability of hazelnut oil and compare it with more common and previously tested olive oil and rapeseed oil. The oxidative stability was determined by means of two independent methods, pressure differential scanning calorimetry (PDSC), and oxidative stability index (OSI) using TA Instruments DSC and Metrohm Rancimat equipment, respectively. Both the PDSC and Rancimat devices were set at five different isothermal temperatures in the range of 100–140 °C. The times to reach peak maximum (τ max) obtained from PDSC exotherms were statistically related with the Rancimat induction times (τ on) to obtain correlation coefficient >0.99 within linear relation. Based on the Arrhenius equation and activated complex theory, activation energies (E a), pre-exponential factors (Z), and specific rate constants (k) for studied oils oxidations were calculated. To the best of our knowledge, this is the first study dealing with oxidative stability of hazelnut oil using PDSC technique. According to the results obtained, PDSC seems to be useful and quick method for kinetic analysis of lipid oxidation in hazelnut oil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tree nuts, including hazelnuts (Corylus avellana L.), are nutritious, provide macronutrients, micronutrients, and bioactive phytochemicals, tasty, convenient, and easy snacks that contribute to healthy lifestyle. Hazelnut oil can be used for several purposed such as cooking, salad dressing, flavouring ingredient, and as well as, a component of skin moisturizers, and cosmetic products [1, 2]. To maintain the freshness of hazelnuts or its oil, it is of paramount importance to study their susceptibility to lipid oxidation in order to optimize the processing and storage conditions.
The assessment of oils and fats quality can be conducted using the oxidative stability determination. Nowadays, the Rancimat Metrohm (Europe) and the oxidative stability index (OSI) (USA) are the most commonly explored and used [3–6]. However, there are also some new methods to evaluate oxidative stability, among others, OXITEST, which enables to assess the oxidation process directly on foodstuff without fat extraction [7], or near infrared emission spectroscopy (NIRES) which allows to determine the induction time of edible oils in accelerated oxidation experiments by monitoring the intensity of a band at 2,900 nm, which corresponds to the formation of hydroperoxides [8].
The transfer of an oxygen molecule to unsaturated fatty acids requires energy. Therefore, the oxidative stability of vegetable oils can also be established by differential scanning calorimetry (DSC) technique, supported by kinetic analysis of lipid oxidation [9]. DSC technique with use of both isothermal (constant temperature) and dynamic (linear increase of temperature) conditions has been successfully applied for analysis of vegetable oils, e.g., rapeseed, olive, soybean, sunflower, and high-stability oils, as reported in literature [4–6, 10–13]. According to Kowalski et al. [10], the isothermal pressure DSC seems to be more suitable than non-isothermal DSC for studying the oxidation of edible oils because the pressure DSC experiment is carried out at lower temperatures, and the heat of transition is more precisely defined.
Few authors [4, 5, 13, 14] who evaluated the oxidative state of oils by DSC highlighted an elevated correlation between thermal analysis and traditional oxidative tests, like oxidative stability instrument or the Rancimat. However, to the best of our knowledge, this is the first study dealing with pressure differential scanning calorimetry (PDSC) and Rancimat in the context of hazelnut oil.
We presumed that PDSC technique alike other methods currently applied for hazelnut oils assessment is fairly useful while have another advantages, e.g., simplicity, quickness and requires quite small amounts of samples for analysis [15]. The main aim of this study was to compare and relate the oxidative stability of hazelnut oils determined by PDSC and the Rancimat at a wide temperature range (100–140 °C). Hence, pave the way for development of PDSC as reliable technique for routine evaluation of oxidative stability of hazelnuts oil and therefore quality of hazelnuts.
Materials and methods
Chemicals
All the solvents (n-hexane, diethyl ether, methanol, ethanol, and chloroform) and reagents (acetic acid, potassium iodide, sodium thiosulfate, potassium hydroxide, starch soluble, and phenolphthalein) used were of analytical grade and purchased from P.O.Ch Co. (Gliwice, Poland).
Materials
Three samples of cold–pressed hazelnut oil (HO), three samples of extra virgin olive oil (OO), and two samples of refined rapeseed oil (RO) were purchased on local market as a food grade commercial products.
Chemical analyses
Peroxide value (PV) of oils was determined by iodometric technique, free fatty acids (% FFA) by titration with 0.1 M ethanolic potassium hydroxide in accordance with Standards ISO 3960:2007 [16], ISO 660:2009 [17], respectively.
Fatty acid analysis
The fatty acid composition of oil samples presented in Table 1 was determined as FAMEs by capillary gas chromatography analysis after alkaline treatment as described by Christie [18]. The chromatographic conditions were the same as reported by Bryś et al. [19].
Pressure differential scanning calorimetry (PDSC)
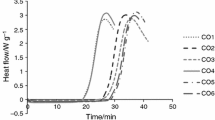
The oxidative stability of tested oils was determined using DSC (Q20, TA Instruments) coupled with a high-pressure cell (Q20P). The equipment was calibrated using high-purity indium as a standard. Oil samples of 3–4 mg were weighted into an aluminium pan and placed in the sample chamber under oxygen atmosphere with an initial pressure of 1,400 kPa and with the 100 mL min−1 oxygen flow rate. The isothermal temperature for each sample was programmed at five different temperatures (100, 110, 120, 130 and 140 °C). Obtained diagrams were analyzed using TA Universal Analysis 2000 software. For each sample, the output was automatically recalculated and presented as amount of energy per 1 g. The maximum PDSC oxidation time (τ max) was determined based on the maximum rate of oxidation (maximum rate of heat flow) with the accuracy of 0.005.
Oxidative stability test
The oxidative stability was determined in 743 Rancimat apparatus from Metrohm according to ISO 6886:1997 [20], utilizing a sample of 2.5 ± 0.01 g. All samples were studied in five temperatures (100, 110, 120, 130, and 140 °C), under a constant air flow (20 L h−1). The induction times were printed automatically by apparatus software with the accuracy of 0.005.
Statistical analysis
Relative standard deviation was obtained, where appropriate, for all data collected. All chemical analyses were carried out in triplicate (n = 3) for each sample. In case of oxidation stability determination, for each oil at each temperature, three experiments were performed and the average τ max (PDSC maximum induction time) and τ on (Rancimat induction time) values were calculated. The data were statistically processed with use of Statgraphics Plus for Windows software, version 4.1 (Statistical Graphics Corporation, Warrenton, VA, USA). Pearson’s linear correlations were calculated at the p < 0.05 level.
Results and discussion
The quality assessment of studied oils
The quality parameters (FFA %, PV) and fatty acids characteristics of the tested oils are gathered in Table 1. The initial characteristics of studied oils show that the oils were of quality with the fatty acids composition of each type of oil within the acceptance range stated in the Codex Alimentarius with regard to fats and oils [21]. The PVs and free fatty acids for HO were significantly lower than for OOs and ROs which suggest that even though HO contains a high amount of unsaturated fatty acids (89.5–90.8 %), the antioxidant (mainly tocopherols) protection works very efficiently. However, the oxidation depends not only on the number of double bonds but also if these bonds are conjugated or not. Unfortunately, it was not measured in present study because not the throughout quality characteristic of studied oils, but the correlation between two methods was the main aim of this paper.
The high tocopherols content with α-tocopherol as a predominant isomer have been reported in hazelnut oils by several authors before [22–25]. Generally, higher level of unsaturation favors oxidation process. The autooxidation rates of oleic, linoleic, and linolenic fatty acids relate to degree of unsaturation and are 1:40:100, respectively [26, 27]. However, the misleading oxidation stability values may be caused by oxidation/hydrolysis products on the one hand, and by endogenous antioxidants present in vegetable oils on the other. It could explain why HOs, which is of higher oleic acid (81.0, 81.2, and 82.1 %), and high tocopherols content [22–25], exhibits significantly higher oxidative stability. Therefore, the chemical composition significantly influences oxidative stability of given oil, which in turn appears to be comprehensive tool in an assessment of the multifaceted oil quality.
PDSC (τ max) and Rancimat (τ on) correlation for hazelnut oil
The results of Rancimat and PDSC measurements for HO, OO, and RO are given in Table 2. For the experiments conducted in the same temperature induction time values obtained with use of PDSC were 50–78 % lower compared to that obtained with the use of Rancimat method. The differences could be related to the smaller sample size used in PDSC measurement (3–4 mg) in comparison to the Rancimat quantity of the sample (2.5 g). According to Tan et al. [14], higher surface-volume ratio of PDSC oil sample also plays an important role leading to shorten the analysis time. Moreover, in contrast to Rancimat, where the air (~21 % O2) and ambient pressure is used, the PDSC analysis is conducted under increased pressure conditions (1,400 kPa) and with the pure oxygen as a gas flow. Therefore, PDSC instrument used to determine oxidative stability works faster than Rancimat which is considerably beneficial in new methodology development.
In order to verify the correlation degree between the two different analytical techniques for hazelnut oil, PDSC and Rancimat, the Pearson linear correlation index was extrapolated for samples tested in five different isothermal temperatures in range of 100–140 °C. The obtained Pearson correlation coefficient value, R > 0.99 for HO samples (Fig. 1), denotes a statistically significant correlation between the two analytical techniques, independent of the temperatures applied (100–140 °C), where the reaction was still of first order. The following correlation Eq. (1) was determined:
In addition to the statistically significant correlation with a well-founded technology as the Rancimat method, the PDSC technique presents several advantages, among which short time of analysis is most important, while some other, such as, smaller size of sample (3–4 mg) compared to Rancimat instrument (2.5 g), and no reagents needed also occur. On the other hand, evident disadvantage of PDSC is use of pure oxygen instead of air.
Kinetic analysis of PDSC and Rancimat data
Kinetic data are essential for prediction the oxidative stability of fats and oils under various heat processing, storage, and distribution conditions [28]. The results of PDSC (τ max) and Rancimat (τ on) were measured as a function of temperature (100–140 °C). For each type of oil studied, the dependence between logarithm of τ max and τ on and temperature was linear with the determination coefficients R 2 > 0.99, while correlation equations were as follows:
where a, b and A, B are adjustable coefficients and t and T are the temperatures expressed in °C, and in K, respectively. From Eq. (2), the average induction times can be calculated for given temperatures for each type of oil separately.
As presented in Figs. 2 and 3, one can notice that three hazelnut oils tested revealed disparate correlations between logarithms of τ max and τ on temperatures. In terms of parameters are listed in Table 1, among the hazelnut oil samples, level of PV for HO1 was significantly lower compared to HO2 and HO3. Low PV together with not examined parameters herein, such as, secondary oxidation products or tocopherols content, in turn could have reflected in higher resistance towards thermo-oxidation of HO1 oil.
Assuming that lipid oxidation of oils in an excess of oxygen is a first-order exothermic process [5, 29, 30], the kinetic parameters, such as, pre-exponential factors (Z) and activation energies (E a) for studied oxidation reactions can be calculated based on the Eq. (3) using A and B coefficients and the Arrhenius equation:
where k is the specific rate constant and R is the gas constant [4, 10]. The results of such calculations along with specific rate constants for HO, OO, and RO are gathered in Table 3.
It was observed that E a varied in the range of 80.99–94.59 and 89.06–92.81 kJ mol−1 for Rancimat and PDSC measurements, respectively. The E a value is affected by the degree of unsaturation of vegetable oil [11, 30]. Adhvaryu et al. [30] reported that a high PUFA content would lower, while high MUFA and SFA content would increase the E a value for lipid oxidation. However, in the present study, no conclusively correlation between fatty acids composition and E a values was observed. The oil oxidation is multifaceted reaction which depends on many factors including fatty acid composition, endogenous antioxidants, catalysts, primary, and secondary oxidation products especially in case of cold-pressed oils.
Considering the rates of lipid oxidation (Table 3) as a function of temperature, an increasing rate of oxidation can be observed when set temperature increases [31]. For example, the rates for HO under the PDSC and Rancimat tests conditions, were 16.1 and 14.1 times higher, respectively, at 140 °C than at 100 °C, what is in a good relation with kinetic theory and data presented previously [4, 9, 10, 28, 31, 32].
Conclusions
To sum up, the preliminary results of PDSC (τ max) and Rancimat (τ on) showed that the oxidative stability of hazelnut oil is similar to the value of olive oil, while higher stability was observed compared to rapeseed oil. Statistically significant linear correlations between PDSC τ max and the Rancimat values imply that PDSC can be recommended as an appropriate objective method for assessing the oxidative stability of hazelnut oils. Additionally, because of the short time of analysis, smaller size of sample, and no reagents needed, the PDSC method has a great potential to be successfully applied and competitive as routine quality control analysis of hazelnut oils.
References
Alasalvar C, Shahidi F. Tree nuts: composition, phytochemicals, and health effects. CRC: Boca Raton; 2008.
Shakerardekani A, Karim R, Ghazali HM, Chin NL. Testural, rheological and sensory properties and oxidative stability of nut spread—a review. Int J Mol Sci. 2013;14:4223–41.
Ratusz K, Kowalski B, Gruczyńska E, Tarnowska K, Bekas W, Kowalska M. Evaluation of antioxidants activity in vegetable oils and triacylglycerol matrices by rancimat measurements. Riv Ital Sostanze Grasse. 2002;79:251–5.
Kowalska D, Kostecka M, Tarnowska K, Kowalski B. Oxidative stabilieties of enzymatically interestrified goose fat and rapeseed oil blend by Rancimat and PDSC. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-013-3125-0.
Kowalski B, Ratusz K, Kowalska D, Bekas W. Determination of oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur J Lipid Sci Technol. 2004;106:165–9.
Farhoosh R. The effect of operational parameters of the Rancimat method on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J Am Oil Chem Soc. 2007;84:205–9.
Comandini P, Verardo V, Maiocchi P, Caboni MF. Accelerated oxidation: comparative study of a new reactor with oxidation stability instrument. Eur J Lipid Sci Technol. 2009;111:933–40.
Vieira FS, Pasquini C. Near infrared emission photometer for measuring the oxidative stability of edible oils. Anal Chim Acta. 2013;796:101–7.
Tan CP, Che Man YB. Recent developments in differential scanning calorimetry for assessing oxidative deterioration of vegetable oils. Trends Food Sci Technol. 2002;13:312–8.
Kowalski B, Gruczyńska E, Maciaszek K. Kinetics of rapeseed oil oxidation by pressure differential scanning calorimetry measurements. Eur J Lipid Sci Technol. 2000;102:337–41.
Kodali DR. Oxidative stability measurement of high stability oils by pressure differential scanning calorimeter (PDSC). J Agric Food Chem. 2005;53:7649–53.
Cordeiro AMTM, Medeiros ML, Silva MAAD, Silva IAA, Soledade LEB, Souza AL, Queiroz N, Souza AG. Rancimat and PDSC accelerated techniques for evaluation of oxidative stability of soybean oil with plant extracts. J Therm Anal Calorim. 2013;114:827–32.
Ostrowska-Ligęza E, Bekas W, Kowalska D, Łobacz M, Wroniak M, Kowalski B. Kinetics of commercial olive oil oxidation: dynamic differential scanning calorimetry and Rancimat studies. Eur J Lipid Sci Technol. 2010;112:268–74.
Tan CP, Che Man YB, Selamat J, Yusoff MSA. Comparative studies of oxidative stability of edible oils by differential scanning calorimetry and oxidative stability index methods. Food Chem. 2002;76:385–9.
Pardauil JJR, Souza LKC, Molfetta FA, Zamian JR, Filho GNR, da Costa CEF. Determination of the oxidative stability by DSC of vegetable oils from the Amazonian area. Bioresour Technol. 2011;102:5873–7.
ISO 3960:2007. Animal and vegetable fats and oils—determination of peroxide value—iodometric (visual) endpoint determination. International Organization for Standardization, Geneva, Switzerland.
ISO 660:2009. Animal and vegetable fats and oils—determination of acid value and acidity. International Organization for Standardization, Geneva, Switzerland.
Christie WW. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J Lipid Res. 1982;23:1072–5.
Bryś J, Wirkowska M, Górska A, Ostrowska-Ligęza E, Bryś A, Koczoń P. The use of DSC and FT-IR spectroscopy for evaluation of oxidative stability of interesterified fats. J Therm Anal Calorim. 2013;112:481–7.
ISO 6886:1997. Animal and vegetable fats and oils—determination of oxidation stability (accelerated oxidation test). International Organization for Standardization, Geneva, Switzerland.
Codex Alimentarius. Joint FAO/WHO food standards programme. Fats, oils and related products, vol. 8. 2nd ed. Rome: Codex Alimentarius; 2001.
Ebrahem KS, Richardson DG, Tetley RM, Mehlenbacher SA. Oil content, fatty acid composition and vitamin E concentration of 17 hazelnut varieties, compared to other types of nuts and oils seeds. Acta Hortic. 1994;351:685–92.
Savage GP, McNeil DL, Dutta PC. Lipid composition and oxidative stability of oils in hazelnuts (Corylus avellana L.) grown in New Zealand. J Am Oil Chem Soc. 1997;74:755–9.
Bada JC, León-Camacho M, Prieto M, Alonso L. Characterization of oils of hazelnuts from Asturias, Spain. Eur J Lipid Sci Technol. 2004;106:294–300.
Kornsteiner M, Wagner KH, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98:381–7.
Arranz S, Cert R, Pérez-Jiménez J, Cert A, Saura-Calixto F. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008;110:985–90.
Frankel EN. Chemistry of free radical and singlet oxidation of lipids. Prog Lipid Res. 1985;23:197–221.
Tan CP, Che Man YB, Selamat J, Yusoff MSA. Application of Arrhenius kinetics to evaluate oxidative stability in vegetable oils by isothermal differential scanning calorimetry. J Am Oil Chem Soc. 2001;11:1133–8.
Litwinienko G, Daniluka A, Kasprzycka-Guttmana T. A differential scanning calorimetry study on the oxidation of C12–C18 saturated fatty acids and their esters. J Am Oil Chem Soc. 1999;76:655–7.
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta. 2000;364:87–97.
Farhoosh R, Niazmand R, Rezaei M, Sarabi M. Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur J Lipid Sci Technol. 2008;110:587–92.
Wirkowska M, Ostrowska-Ligęza E, Górska A, Koczoń P. Thermal properties of fats extracted from powdered baby formulas. J Therm Anal Calorim. 2012;110:137–43.
Acknowledgements
This publication has been co-financed with the European Union funds by the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ciemniewska-Żytkiewicz, H., Ratusz, K., Bryś, J. et al. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J Therm Anal Calorim 118, 875–881 (2014). https://doi.org/10.1007/s10973-014-3861-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3861-9