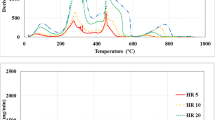
Abstract
The hyphenated thermal analysis-mass spectrometry technique (TA-MS) was applied for the investigation of the thermal behavior of reference and aged parchment samples. The kinetic parameters of the process were calculated independently from all recorded TA and MS signals. The kinetic analysis showed the distinct dependence of the activation energy on the reaction progress. Such behavior is characteristic for the multistage mechanism of the reaction.
The comparison of the kinetic parameters calculated from the different signals i.e. TG, DSC, MS for H2O, NO and CO2, however, indicated that they were differently dependent on the aging of the sample. For the parchment samples, the aging almost does not change the kinetics of the decomposition calculated from the DSC data: the influence of aging seems to be too negligible to be detected by these techniques. On the other hand, the much more sensitive mass spectrometric technique applied to the kinetic analysis allowed monitoring of visible changes in the thermal behavior of the parchment samples due to the aging process. The influence of aging was especially visible when the MS signals of water and nitric oxide were applied for the determination of the kinetic parameters.
The applied method of the kinetic analysis allowed also the prediction of the thermal behaviour of reference and aged parchment samples under isothermal and modulated temperature conditions. Presented results have confirmed the usefulness of thermoanalytical methods for investigating behaviour of such complicated systems as leather or parchment.
Similar content being viewed by others
References
Advanced Kinetics and Technology Solutions: http://www.akts.com (AKTS-Thermokinetics software and AKTS-Thermal Safety software).
NS Cohen M Odlyha GM Foster (2000) Thermochim. Acta 365 111 Occurrence Handle1:CAS:528:DC%2BD3cXosFaqsb8%3D Occurrence Handle10.1016/S0040-6031(00)00618-3
B Roduit J Baldyga M Maciejewski A Baiker (1997) Thermochim. Acta 295 59 Occurrence Handle1:CAS:528:DyaK2sXksl2ktbs%3D Occurrence Handle10.1016/S0040-6031(97)00101-9
ME Brown M Maciejewski S Vyazovkin R Nomen J Sempere A Burnham J Opfermann R Strey HL Anderson A Kemmler R Keuleers J Janssens HO Desseyn C-R Li TB Tang B Roduit J Malek T Mitsuhashi (2000) Thermochim. Acta 355 125 Occurrence Handle1:CAS:528:DC%2BD3cXktVSjsr0%3D Occurrence Handle10.1016/S0040-6031(00)00443-3
M Maciejewski (2000) Thermochim. Acta 355 145 Occurrence Handle1:CAS:528:DC%2BD3cXktVSjsro%3D Occurrence Handle10.1016/S0040-6031(00)00444-5
A Burnham (2000) Thermochim. Acta 355 165 Occurrence Handle1:CAS:528:DC%2BD3cXktVSjsrg%3D Occurrence Handle10.1016/S0040-6031(00)00446-9
B Roduit (2000) Thermochim. Acta 355 171 Occurrence Handle1:CAS:528:DC%2BD3cXktVSjsrk%3D Occurrence Handle10.1016/S0040-6031(00)00447-0
B Roduit Ch Borgeat B Berger P Folly B Alonso JN Aebischer (2005) J. Therm. Anal. Cal. 80 91 Occurrence Handle1:CAS:528:DC%2BD2MXktl2ksL4%3D Occurrence Handle10.1007/s10973-005-0619-4
B Roduit Ch Borgeat B Berger P Folly B Alonso JN Aebischer F Stoessel (2005) J. Therm. Anal. Cal. 80 229 Occurrence Handle1:CAS:528:DC%2BD2MXktl2lu7Y%3D Occurrence Handle10.1007/s10973-005-0641-6
WF Hemminger SM Sarge (1991) J. Thermal Anal. 37 1455 Occurrence Handle1:CAS:528:DyaK38Xpt1Gqtg%3D%3D Occurrence Handle10.1007/BF01913481
HL Friedman (1966) J. Polym. Lett. 4 323 Occurrence Handle10.1002/pol.1966.110040504
T Ozawa (1965) Bull. Chem. Soc. Jpn. 38 1881 Occurrence Handle1:CAS:528:DyaF28XjtVyisQ%3D%3D Occurrence Handle10.1246/bcsj.38.1881
JH Flynn LA Wall (1966) J. Res. Nat. Bur. Standards 70A 487
P Budrugeac (2002) J. Therm. Anal. Cal. 68 131 Occurrence Handle1:CAS:528:DC%2BD38XjvFKgurs%3D Occurrence Handle10.1023/A:1014932903582
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roduit, B., Odlyha, M. Prediction of thermal stability of fresh and aged parchment. J Therm Anal Calorim 85, 157–164 (2006). https://doi.org/10.1007/s10973-005-7410-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7410-4