Abstract
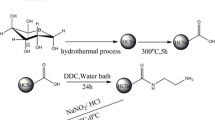
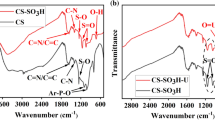
The thiol-functional hydrotalcite (Mg/Al-LDO-SH) composite materials were prepared and characterized by EDS, SEM, FT-IR and XRD. The variables influencing the adsorption capacity were investigated. Results show that 3-MPTMS got success in material surface modification. The best optimization condition for adsorption experiment was at time 150 min, pH 3.0, temperature 30 °C, initial uranium (VI) concentration 30 mg L−1, adsorption dosage 10 mg with 99.06% of adsorption efficiency and 1545.32 mg g−1 of adsorption capacity. Kinetics data follow the pseudo-second-order model and equilibrium data fit the Freundlich isotherm model very well. Thermodynamic studies show that adsorption process is spontaneous and exothermic.

















Similar content being viewed by others
References
Craft E, Abu-Qare A, Flaherty M, Garofolo M, Rincavage H, Abou-Donia M (2004) Depleted and natural uranium: chemistry and toxicological effects. J Toxicol Environ Health Part B Crit Rev 7(4):297–317. https://doi.org/10.1080/10937400490452714
Duan C, Huo J, Li F, Yang M, Xi H (2018) Ultrafast room-temperature synthesis of hierarchically porous metal–organic frameworks by a versatile cooperative template strategy. J Mater Sci 53:16276–16287
Li Z, Chen F, Yuan L, Liu Y, Zhao Y, Chai Z, Shi W (2012) Uranium (VI) adsorption on graphene oxide nanosheets from aqueous solutions. Chem Eng J 210(6):539–546
Zhang A, Asakura T, Uchiyama G (2003) The adsorption mechanism of uranium (VI) from seawater on a macroporous fibrous polymeric adsorbent containing amidoxime chelating functional group. React Funct Polym 57(1):67–76
Massarin S, Beaudouin R, Zeman F, Floriani M, Gilbin R, Alonzo F, Pery ARR (2011) Biology-based modeling to analyze uranium toxicity data on Daphnia magna in a multigeneration study. Environ Sci Technol 45(9):4151
Chapman N, Hooper A (2012) The disposal of radioactive wastes underground. Proc Geol Assoc 123(1):46–63
Garcã-A-Balboa C, Baselga-Cervera B, Garcã-A-Sanchez A, Igual JM, Lopez-Rodas V, Costas E (2013) Rapid adaptation of microalgae to bodies of water with extreme pollution from uranium mining: an explanation of how mesophilic organisms can rapidly colonise extremely toxic environments. Aquatic Toxicol s 144–145:116–123
Kuncham K, Nair S, Durani S, Bose R (2017) Efficient removal of uranium (VI) from aqueous medium using ceria nanocrystals: an adsorption behavioural study. J Radioanal Nucl Chem 313(1):1–12
Tripathi S, Roy A, Nair S, Durani S, Bose R (2018) Removal of U(VI) from aqueous solution by adsorption onto synthesized silica and zinc silicate nanotubes: equilibrium and kinetic aspects with application to real samples. Environ Nanotechnol Monit Manag 10
Metilda P, Sanghamitra K, Gladis JM, Naidu GRK, Rao TP (2005) Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium (VI). Talanta 65(1):192–200
Venkatesan KA, Shyamala KV, Antony MP, Srinivasan TG, Rao PRV (2008) Batch and dynamic extraction of uranium (VI) from nitric acid medium by commercial phosphinic acid resin, Tulsion CH-96. J Radioanal Nucl Chem 275(3):563–570
Saini AS, Melo JS (2013) Biosorption of uranium by melanin: kinetic, equilibrium and thermodynamic studies. Bioresour Technol 149(12):155–162
Liu N, Wang Y, He C (2016) Tetraphenylimidodiphosphinate as solid phase extractant for preconcentrative separation of thorium from aqueous solution. J Radioanal Nucl Chem 308(2):393–401
Lemons B, Khaing H, Ward A, Thakur P (2018) A rapid method for the sequential separation of polonium, plutonium, americium and uranium in drinking water. Appl Radiat Isot 136:10–17
Karadağ E, Saraydin D, Güven O (1995) Behaviors of acrylamide/itaconic acid hydrogels in uptake of uranyl ions from aqueous solutions. Sep Sci 30(20):3747–3760
Nogami M, Sugiyama Y, Kawasaki T, Harada M, Morita Y, Kikuchi T, Ikeda Y (2010) Adsorptivity of polyvinylpolypyrrolidone for selective separation of U(VI) from nitric acid media. J Radioanal Nucl Chem 283(2):541–546
Özeroğlu C, Metin N (2012) Adsorption of uranium ions by crosslinked polyester resin functionalized with acrylic acid from aqueous solutions. J Radioanal Nucl Chem 292(2):923–935
Haque E, Lee JE, Jang IT, Hwang YK, Chang JS, Jegal J, Jhung SH (2010) Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J Hazard Mater 181(1):535–542
Zhang B, Li F, Wu T, Sun D, Li Y (2015) Adsorption of p-nitrophenol from aqueous solutions using nanographite oxide. Colloids Surf A 464:78–88
Özeroğlu C, Doğan E, Keçeli G (2011) Investigation of Cs(I) adsorption on densely crosslinked poly(sodium methacrylate) from aqueous solutions. J Radioanal Nucl Chem 289(2):577–586
Özeroğlu C, Bilgiç ÖD (2015) Use of the crosslinked copolymer functionalized with acrylic acid for the removal of strontium ions from aqueous solutions. J Radioanal Nucl Chem 305(2):551–565
Chen S, Hong J, Yang H, Yang J (2013) Adsorption of uranium (VI) from aqueous solution using a novel graphene oxide-activated carbon felt composite. J Environ Radioact 126(4):253–258
Gao MW, Zhu GR, Wang XH, Wang P, Gao CJ (2015) Preparation of short channels SBA-15-PVC membrane and its adsorption properties for removal of uranium (VI). J Radioanal Nucl Chem 304(2):675–682
Cheng W, Wan T, Wang X, Wu W, Hu B (2018) Plasma-grafted polyamine/hydrotalcite as high efficient adsorbents for retention of uranium (VI) from aqueous solutions. Chem Eng J 342
Yao W, Wang XX, Liang Y, Yu SJ, Gu PC, Sun YB, Xu C, Chen J, Hayat T, Alsaedi A, Wang XK (2018) Synthesis of novel flower-like layered double oxides/carbon dots nanocomposites for U(VI) and Am-241(III) efficient removal: batch and EXAFS studies. Chem Eng J 332:775–786. https://doi.org/10.1016/j.cej.2017.09.011
Wang F, Liu Q, Li R, Li Z, Zhang H, Liu L, Wang J (2016) Selective adsorption of uranium (VI) onto prismatic sulfides from aqueous solution. Colloids Surf A 490:215–221
Liao Y, Wang M, Chen D (2018) Production of three-dimensional porous polydopamine-functionalized attapulgite/chitosan aerogel for uranium (VI) adsorption. J Radioanal Nucl Chem 316(2):1–13
Chitrakar R, Tezuka S, Sonoda A, Sakane K, Ooi K, Hirotsu T (2005) Adsorption of phosphate from seawater on calcined MgMn-layered double hydroxides. J Colloid Interface Sci 290(1):45–51
Das J, Patra BS, Baliarsingh N, Parida KM (2006) Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl Clay Sci 32(3–4):252–260
Garciagallastegui A, Iruretagoyena D, Gouvea V, Mokhtar M, Asiri AM, Basahel SN, Althabaiti SA, Alyoubi AO, Chadwick D, Shaffer MSP (2012) Graphene oxide as support for layered double hydroxides: enhancing the CO2 adsorption capacity. Chem Mater 24(23):4531–4539
Tan L, Wang Y, Liu Q, Wang J, Jing X, Liu L, Liu J, Song D (2015) Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem Eng J 259((Complete)):752–760
Guo-Jun KE, Zhang L, Yang PF, Zhao HD, Tan HJ (2017) Controlled synthesis of Mg-Al hydrotalcites with different morphologies and their adsorption performances for chloride ion. Fine Chem
Zhang S, Zhang Y, Liu J, Xu Q, Xiao H, Wang X, Xu H, Zhou J (2013) Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem Eng J 226(24):30–38
Lu X, Yin Q, Xin Z, Zhang Z (2010) Powerful adsorption of silver (I) onto thiol-functionalized polysilsesquioxane microspheres. Chem Eng Sci 65(24):6471–6477
Fang L, Hou J, Xu C, Wang Y, Li J, Xiao F, Wang D (2018) Enhanced removal of natural organic matters by calcined Mg/Al layered double hydroxide nanocrystalline particles: adsorption, reusability and mechanism studies. Appl Surf Sci 442:45–53
Kalin M, Wheeler WN, Meinrath G (2004) The removal of uranium from mining waste water using algal/microbial biomass. J Environ Radioact 78(2):151–177
Tong KS, Kassim MJ, Azraa A (2011) Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: equilibrium, kinetics, and thermodynamic studies. Chem Eng J 170(1):145–153
Atia AA (2005) Studies on the interaction of mercury (II) and uranyl (II) with modified chitosan resins. Hydrometallurgy 80(1–2):13–22
Li W, Tao Z (2002) Comparative study on Th(IV) sorption on alumina and silica from aqueous solutions. J Radioanal Nucl Chem 254(1):187–192
Fardmousavi O, Faghihian H (2014) Thiol-functionalized hierarchical zeolite nanocomposite for adsorption of Hg2+ from aqueous solutions. C R Chim 17(12):1203–1211
Zhou L, Shang C, Liu Z, Huang G, Adesina AA (2012) Selective adsorption of uranium (VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366(1):165–172
Wang Z, Yao Q, Liu P, Li X, Yuan (2011) Kinetics and thermodynamics for acid red 88 adsorption on calcined layered double hydroxides. Acta Chim Sinica 69(5):529–535
Li Y, Wang J, Li Z, Liu Q, Liu J, Liu L, Zhang X, Yu J (2013) Ultrasound assisted synthesis of Ca–Al hydrotalcite for U(VI) and Cr(VI) adsorption. Chem Eng J 218(3):295–302
Acknowledgements
This study was financially supported by the Natural Science Foundation of Hunan Province (2017JJ2231).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any possible conflicts of interest.
Rights and permissions
About this article
Cite this article
Xu, Y., Ke, G., Yin, J. et al. Synthesis of thiol-functionalized hydrotalcite and its application for adsorption of uranium (VI). J Radioanal Nucl Chem 319, 791–803 (2019). https://doi.org/10.1007/s10967-018-6376-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6376-1