Abstract
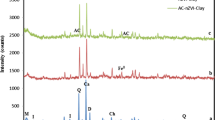
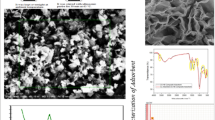
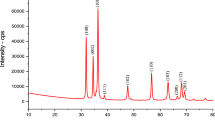
In this study, polyaniline/Clay nanomaterials (PANI/Clay) was synthesized through one-step method and used as an adsorbent to remove Cu(II) ions from aqueous solution. The PANI/Clay was characterized using X-ray diffraction, Fourier transform infrared spectroscopy, thermogravimetric analysis, cyclic voltammograms and physical adsorption of gases. Adsorption parameters such as contact time, pH value, and initial metal ion concentration were investigated. The results revealed that PANI/Clay exhibits a much higher adsorption capacity than the natural clay; the attractive adsorption capacity reached 22.77 mg/g with 0.05 g of 100 mg PANI/Clay at an initial pH solution of 6 and adsorption temperature of 25 °C. Moreover, the Langmuir model well describes the adsorption data with the maximum sorption capacity of 22.77 mg/g. Pseudo-second-order model can fit well the kinetic data obtained from batch Cu(II) removal experiments. The Cu(II) adsorption on PANI/Clay nanocomposite was mainly attributed to electrostatic interaction, donor–acceptor interaction and intermolecular interactions.









Similar content being viewed by others
References
P.J. Collins, M.J. Kotterman, J.A. Field, A.D. Dobson, Oxidation of anthracene and benzo[a]pyrene by laccases from trametes versicolor. Appl. Environ. Microbiol. 62, 4563–4567 (1996)
A. Majcherczyk, C. Johannes, A. Hüttermann, Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of trametes versicolor. Enzym. Microb. Technol. 22, 335–341 (1998)
H. Ali, Biodegradation of synthetic dyes-a review. Water Air Soil Pollut. 213, 251–273 (2010)
E. Guibal, J. Roussy, Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan). React. Funct. Polym. 67, 33–42 (2007)
H. Xu, D.D.L. Liu, L. He, Adsorption of Copper(II) from an wastewater effluent of electroplating industry by poly(ethyleneimine)-functionalized silica. Iran. J. Chem. Chem. Eng. 34, 73–81 (2015)
S.H. Ahmadi, P. Davar, A. Manbohi, Adsorptive removal of reactive orange 122 from aqueous solutions by ionic liquid coated Fe3O4 magnetic nanoparticles as an efficient adsorbent. Iran. J. Chem. Chem. Eng. 35, 63–73 (2016)
V.K. Gupta, R. Jain, S. Varshney, Electrochemical removal of the hazardous dye reactofix red 3 BFN from industrial effluents. J. Colloid Interface Sci. 312, 292–296 (2007)
M. Ishaq, K. Saee, I. Ahmad, S. Sultan, Coal ash as a low cost adsorbent for the removal of xylenol orange from aqueous solution. Iran. J. Chem. Chem. Eng. 33, 53–58 (2014)
K.Y. Foo, B.H. Hameed, Preparation of activated carbon from date stones by microwave induced chemical activation: application for methylene blue adsorption. Chem. Eng. J. 170, 338–341 (2011)
R. Wang, R. Yang, Y. Zhang, A study of applying green glucose-reduced graphene oxide in advanced treatment of different dyes. Desalin. Water Treat. 70, 387–393 (2017)
Y.E. Miao, R. Wang, D. Chen, Z. Liu, T. Liu, Electrospun self-standing membrane of hierarchical SiO2@γ-AlOOH (Boehmite) core/sheath fibers for water remediation. ACS Appl. Mater. Interfaces 4, 5353–5359 (2012)
M.M. Ayad, A.A. El-Nasr, Adsorption of cationic dye (methylene blue) from water using polyaniline nanotubes base. J. Phys. Chem. C 114, 14377–14383 (2010)
V. Janaki, B.T. Oh, K. Shanthi, K.J. Lee, A.K. Ramasamy, S.K. Kannan, Polyaniline/chitosan composite: an eco-friendly polymer for enhanced removal of dyes from aqueous solution. Synth. Met. 162, 974–980 (2012)
V. Janaki, K. Vijayaraghavan, B.T. Oh, K. Shanthi, K.J. Lee, A.K. Ramasamy, Synthesis, characterization and application of cellulose/polyaniline nanocomposite for the treatment of simulated textile effluent. Cellulose 20, 1153–1166 (2013)
S. Zhang, L. Gao, L. Shan, R. Wang, Y. Min, Comparative study on the adsorption of NO2 using different clay/polyaniline composites. Ind. Eng. Chem. Res. 57, 6897–6903 (2018)
V. Janaki, K. Vijayaraghavan, A.K. Ramasamy, K.J. Lee, B.T. Oh, S.K. Kannan, Competitive adsorption of reactive orange 16 and reactive brilliant blue R on polyaniline/bacterial extracellular polysaccharides composite-A novel eco-friendly polymer. J. Hazard. Mater. 241–242, 110–117 (2012)
R. Karthik, S. Meenakshi, Facile synthesis of cross linked-chitosan-grafted polyaniline composite and its Cr(VI) uptake studies. Int. J. Biol. Macromol. 67, 210–219 (2014)
Y. Lei, X. Qian, J. Shen, X. An, Integrated reductive/adsorptive detoxification of Cr(VI)-contaminated water by polypyrrole/cellulose fiber composite. Ind. Eng. Chem. Res. 51, 10408–10415 (2012)
R. Karthik, S. Meenakshi, Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 263, 168–177 (2015)
R. Karthik, S. Meenakshi, Synthesis, characterization and Cr(VI) uptake study of polyaniline coated chitin. Int. J. Biol. Macromol. 72, 235–242 (2015)
S. Larous, A.H. Meniai, Removal of copper(II) from aqueous solution by agricultural by-products sawdust. Energy Proced. 18, 915–923 (2012)
E. Igberase, P. Osifo, A. Ofomaja, The adsorption of copper(II) ions by polyaniline graft chitosan beads from aqueous solution: equilibrium, kinetic and desorption studies. J. Environ. Chem. Eng. 2, 362–369 (2014)
X. Xue, F. Li, Removal of Cu(II) from aqueous solution by adsorption onto functionalized SBA-16 mesoporous silica. Microporous Mesoporous Mater. 116, 116–122 (2008)
X. Zhang, Q. Huang, M. Liu, J. Tian, G. Zeng, Z. Li, K. Wang, Q. Zhang, Q. Wan, F. Deng, Y. We, Preparation of amine functionalized carbon nanotubes via a bioinspired strategy and their application in Cu2 + removal. Appl. Surf. Sci. 343, 19–27 (2015)
Y. Xie, Q. Huang, M. Liu, K. Wang, Q. Wan, F. Deng, L. Lu, X. Zhang, Y. Wei, Mussel inspired functionalization of carbon nanotubes for heavy metal ion removal. RSC Adv. 5, 68430–68438 (2015)
X. Zhang, Q. Huang, F. Deng, H. Huang, Q. Wan, M. Liu, Y. Wei, Mussel-inspired fabrication of functional materials and their environmental applications: progress and prospects. Appl. Mater. Today 7, 222–238 (2017)
W. Jiang, W. Wang, B. Pan, Q. Zhang, W. Zhang, L. Lv, Facile fabrication of magnetic chitosan beads of fast kinetics and high capacity for copper removal. ACS Appl. Mater. Interfaces 6, 3421–3426 (2014)
Q. Huang, M. Liu, J. Chen, K. Wang, D. Xu, F. Deng, H. Huang, X. Zhang, Y. Wei, Mussel inspired preparation of functional silica nanocomposites for environmental adsorption applications. Appl. Surf. Sci. 387, 285–293 (2016)
X. Zhang, K. Wang, M. Liu, X. Zhang, L. Tao, Y. Chen, Y. Wei, Polymeric AIE-based nanoprobes for biomedical applications: recent advances and perspectives. Nanoscale 7, 11486–11508 (2015)
Q. Wan, M. Liu, Y. Xie, J. Tian, Q. Huang, F. Deng, L. Mao, Q. Zhang, X. Zhang, Y. Wei, Facile and highly efficient fabrication of graphene oxide-based polymer nanocomposites through mussel-inspired chemistry and their environmental pollutant removal application. J. Mater. Sci. 52, 504–518 (2017)
Q. Huang, M. Liu, J. Chen, Q. Wan, J. Tian, L. Huang, R. Jiang, Y. Wen, X. Zhang, Y. Wei, Facile preparation of MoS2 based polymer composites via mussel inspired chemistry and their high efficiency for removal of organic dyes. Appl. Surf. Sci. 419, 35–44 (2017)
A. Zehhaf, A. Benyoucef, R. Berenguer, C. Quijada, S. Taleb, E. Morallon, Lead ion adsorption from aqueous solutions in modified Algerian Montmorillonites. J. Therm. Anal. Calorim. 110, 1069–1077 (2012)
I. Toumi, A. Benyoucef, A. Yahiaoui, C. Quijada, E. Morallon, Effect of the intercalated cation-exchanged on the properties of nanocomposites prepared by 2-aminobenzene sulfonic acid with aniline and montmorillonite. J. Alloy. Compd. 551, 212–218 (2013)
A. Zehhaf, A. Benyoucef, C. Quijada, S. Taleb, E. Morallon, Algerian natural montmorillonites for arsenic(III) removal in aqueous solution. Int. J. Environ. Sci. Technol. 12, 595–602 (2015)
I. Ali, M. Asim, T.A. Khan, Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 113, 170–183 (2012)
F. Chouli, I. Radja, E. Morallon, A. Benyoucef, A Novel conducting nanocomposite obtained by p-anisidine and aniline with titanium(IV) oxide nanoparticles: synthesis, characterization, and electrochemical properties. Polym. Compos. 38, 254–260 (2017)
M. Mekhloufi, A. Zehhaf, A. Benyoucef, C. Quijada, E. Morallon, Removal of 8-quinolinecarboxylic acid pesticide from aqueous solution by adsorption on activated montmorillonites. Environ. Monit. Assess. 185, 10365–10375 (2013)
S.J. Gregg, K.S.W. Sing, Adsorption, Surface Area and Porosity, 2nd edn. (Academic Press, London, 1982), pp. 3–12
P.G. Ingole, R.R. Pawar, M.I. Baig, J.D. Jeon, H.K. Lee, Thin film nanocomposite (TFN) hollow fiber membranes incorporated with functionalized acid-activated bentonite (ABn-NH) clay: towards enhancement of water vapor permeance and selectivity. J. Mater. Chem. A 5, 20947–20958 (2017)
B.H. Kim, J.H. Jung, J.W. Kim, H.J. Choi, J. Joo, Nanocomposite intercalated by emulsion polymerization. Synth. Met. 117, 115–118 (2001)
A. Guinier, X-ray Diffraction in Crystals, Imperfect Crystals and Amorphous Bodies (Freeman & Co., San Francisco, 1994)
Y.P. Chang, C.L. Ren, J.C. Qu, X.G. Chen, Preparation and characterization of Fe3O4/graphene nanocomposite and investigation of its adsorption performance for aniline and p-chloroaniline. Appl. Surf. Sci. 261, 504–509 (2012)
J. Wang, L. Bi, Y. Ji, H. Ma, X. Yin, Removal of humic acid from aqueous solution by magnetically separable polyaniline: adsorption behavior and mechanism. J. Colloid Interface Sci. 430, 140–146 (2014)
P.D. Saha, S. Chakraborty, S. Chowdhury, Batch and continuous (fixed-bed column) biosorption of crystal violet by Artocarpusheterophyllus (jackfruit) leaf powder. Colloids Surf. B 92, 262–270 (2012)
C.M. Yon, J.D. Sherman, Adsorption. In Gas Separation. Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 1, (Wiley, New york, 2003)
H.J. Kim, S. Im, J.C. Kim, W.G. Hong, K. Shin, H.Y. Jeong, Y.J. Hong, Phytic acid doped polyaniline nanofibers for enhanced aqueous copper(ii) adsorption capability. ACS Sustain. Chem. Eng. 5, 6654–6664 (2017)
N. Jiang, Y. Xu, Y. Dai, W. Luo, L. Dai, Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. J. Hazard. Mater. 215–216, 17–24 (2012)
S. Benyakhou, A. Belmokhtar, A. Zehhaf, A. Benyoucef, Development of novel hybrid materials based on poly(2-aminophenyl disulfide)/Silica Gel: preparation, characterization and electrochemical studies. J. Mol. Struct. 1150, 580–585 (2017)
P. Baroni, R.S. Vieira, E. Meneghetti, M.G.C. da Silva, M.M. Beppu, Evaluation of batch adsorption of chromium ions on natural and crosslinked chitosan membranes. J. Hazard. Mater. 152, 1155–1163 (2008)
Acknowledgements
The authors would like to thank the General Management of Scientific Research and Technological Development (DGRSDT) Algeria. Prof. Abdelghani Benyoucef would like to thank Prof Emilia Morallon of Instituto Universitario de Materiales, Alicante University (Spain) for the analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soltani, H., Belmokhtar, A., Zeggai, F.Z. et al. Copper(II) Removal from Aqueous Solutions by PANI-Clay Hybrid Material: Fabrication, Characterization, Adsorption and Kinetics Study. J Inorg Organomet Polym 29, 841–850 (2019). https://doi.org/10.1007/s10904-018-01058-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-01058-z