Abstract
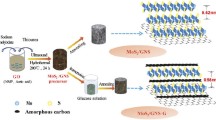
Molybdenum disulphide, a two dimensional unique layered material with natural hexagonal structure with adjoining layers connected by Mo–S covalent bonds, and stacked by weak van der Waals forces is similar to graphite. It has adjoining layer spacing of 0.615 nm which is significantly wider than graphite (0.333 nm) and is fabricated by simple inert annealing process. The synthesized MoS2 is composited with reduced graphene oxide sheets in 1:1 ratio to enhance its electrochemical performance by improving electrical conductivity. MoS2–rGO nanocomposite manifested a high specific discharge capacity of 1523 mAh g−1 at 70 mA g−1 and sustained a capacity of 1270 mAh g−1 at the end of 200 charge–discharge cycles demonstrating an excellent stability of the material. Nevertheless, the nanocomposite material also exhibited phenomenal rate capability by displaying specific capacities of 400 and 300 mAh g−1 at high current densities of 700 and 1400 mA g−1, respectively.











Similar content being viewed by others
References
L. Hu, Y. Ren, H. Yang, Q. Xu, ACS Appl. Mater. Interfaces 6, 14644–14652 (2014)
U.K. Sen, S. Mitra, ACS Appl. Mater. Interfaces 5, 1240–1247 (2013)
S.K. Das, R. Mallavajula, N. Jayaprakash, L.A. Archer, J. Mater. Chem. 22, 12988–12992 (2012)
Y. Lu, X. Yao, J. Yin, G. Peng, P. Cui, X. Xu, RSC Adv. 5, 7938–7943 (2015)
Y. Wang, L. Yu, X.W. Lou, Angew. Chem. Int. Ed. 55, 7423–7426 (2016)
X. Zuo, K. Chang, J. Zhao, Z. Xie, H. Tang, B. Li, Z. Chang, J. Mater. Chem. A 4, 51–58 (2016)
C. Zhu, X. Mu, P.A. van Aken, J. Maier, Y. Yu, Adv. Energy Mater. 5, 1401170–1401178 (2015)
J. Zhou, J. Qin, X. Zhang, C. Shi, E. Liu, J. Li, N. Zhao, C. He, ACS Nano 9, 3837–3848 (2015)
C. Zhu, X. Mu, P.A. van Aken, Y. Yu, J. Maier, Angew. Chem. 126, 2184–2188 (2014)
H. Jiang, D. Ren, H. Wang, Y. Hu, S. Guo, H. Yuan, P. Hu, L. Zhang, C. Li, Adv. Mater. 27, 3687–3695 (2015)
S. Guo, Q. Zhang, Z. Zhu, J. Xie, J. Fan, Q. Xu, P. Shi, Y. Min, Chem. Sel. 2, 3117–3128 (2017)
Z.-H. Miao, P.-P. Wang, Y.-C. Xiao, H.-T. Fang, L. Zhen, C.-Y. Xu, ACS Appl Mater Interfaces 8, 33741–33748 (2016)
B. Hou, X. Wang, J. Yao, H. Zhang, W. Yu, G. Liu, X. Dong, L. Wang, J. Wang, J. Mater. Sci. 28, 12297–12305 (2017)
W.S. Hummers Jr., R.E. Offeman, J. Am. Chem. Soc. 80, 1339–1339 (1958)
M.S. Raghu, K.Y. Kumar, S. Rao, T. Aravinda, S.C. Sharma, M.K. Prashanth, Phys. B 537, 336–345 (2018)
S.B. Patil, B. Kishore, M.K. Nagaraj, N. Ganganagappa, U. Velu, Chem. Sel. 3, 7490–7495 (2018)
S.B. Patil, T.N. Ravishankar, K. Lingaraju, G.K. Raghu, G. Nagaraju, J. Mater. Sci. 29, 277–287 (2018)
L. Wang, J. Li, H. Zhou, Z. Huang, B. Zhai, L. Liu, L. Hu, J. Mater. Sci. 29, 3110–3119 (2018)
S.K. Srivastava, B. Kartick, S. Choudhury, M. Stamm, Mater. Chem. Phys. 183, 383–391 (2016)
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, S. Sivaramakrishnan, J. Phys. Chem. Solids 112, 106–118 (2018)
V. Revathi, K. Karthik, J. Mater. Sci. 29, 18519–18530 (2018)
K.S.W. Sing, Pure Appl. Chem. 57, 603–619 (1985)
X. Hu, Y. Li, G. Zeng, J. Jia, H. Zhan, Z. Wen, ACS Nano 12, 1592–1602 (2018)
N. Lingappan, D.J. Kang, Electrochim. Acta 193, 128–136 (2016)
J.-G. Wang, R. Zhou, D. Jin, K. Xie, B. Wei, Electrochim. Acta 231, 396–402 (2017)
S. Xia, Y. Wang, Y. Liu, C. Wu, M. Wu, H. Zhang, Chem. Eng. J. 332, 431–439 (2018)
Y. Zhong, Q. Zhuang, C. Mao, Z. Xu, Z. Guo, G. Li, J. Alloys Compd. 745, 8–15 (2018)
M. Choi, J. Hwang, H. Setiadi, W. Chang, J. Kim, J. Supercritical Fluids 127, 81–89 (2017)
Z. Wang, S. Madhavi, X.W. Lou, J. Phys. Chem. C 116, 12508–12513 (2012)
F. Xiong, Z. Cai, L. Qu, P. Zhang, Z. Yuan, O.K. Asare, W. Xu, C. Lin, L. Mai, ACS Appl. Mater. Interfaces 7, 12625–12630 (2015)
Acknowledgements
GN and SBP greatly thank BRNS-BARC, DAE (No.: 37(2)/14/25/2015/BRNS) Bombay, Govt. of India for financial sponsorship. Thanks to CoE - TEQIP, Director and Principal, Siddaganga Institute of Technology (SIT), Tumakuru for constant support and encouragement. Thanks to Prof. N. Munichandraiah, Dept. of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore for providing glove box facility to assemble the cells.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, S.B., Raghu, M.S., Kishore, B. et al. Enhanced electrochemical performance of few-layered MoS2–rGO nanocomposite for lithium storage application. J Mater Sci: Mater Electron 30, 316–322 (2019). https://doi.org/10.1007/s10854-018-0295-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0295-3