Abstract
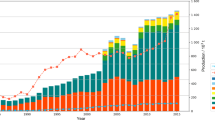
Seaweed aquaculture beds (SABs) that support the production of seaweed and their diverse products, cover extensive coastal areas, especially in the Asian-Pacific region, and provide many ecosystem services such as nutrient removal and CO2 assimilation. The use of SABs in potential carbon dioxide (CO2) mitigation efforts has been proposed with commercial seaweed production in China, India, Indonesia, Japan, Malaysia, Philippines, Republic of Korea, Thailand, and Vietnam, and is at a nascent stage in Australia and New Zealand. We attempted to consider the total annual potential of SABs to drawdown and fix anthropogenic CO2. In the last decade, seaweed production has increased tremendously in the Asian-Pacific region. In 2014, the total annual production of Asian-Pacific SABs surpassed 2.61 × 106 t dw. Total carbon accumulated annually was more than 0.78 × 106 t y−1, equivalent to over 2.87 × 106 t CO2 y−1. By increasing the area available for SABs, biomass production, carbon accumulation, and CO2 drawdown can be enhanced. The conversion of biomass to biofuel can reduce the use of fossil fuels and provide additional mitigation of CO2 emissions. Contributions of seaweeds as carbon donors to other ecosystems could be significant in global carbon sequestration. The ongoing development of SABs would not only ensure that Asian-Pacific countries will remain leaders in the global seaweed industry but may also provide an added dimension of helping to mitigate the problem of excessive CO2 emissions.
Similar content being viewed by others
Change history
25 April 2017
An erratum to this article has been published.
References
Adame MF, Wright SF, Grinham A, Lobb K, Reymond CE, Lovelock CE (2012) Terrestrial-marine connectivity: patterns of terrestrial soil carbon deposition in coastal sediments determined by analysis of glomalin related soil protein. Limnol Oceanogr 57:1492–1502
Adams JMM, Schmidt A, Gallagher JA (2015) The impact of sample preparation of the macroalgae Laminaria digitata on the production of the biofuels bioethanol and biomethane. J Appl Phycol 27:985–991
AGEDI (2013) Blue carbon in Abu Dhabi. Protecting our coastal heritage: The Abu Dhabi clue carbon demonstration project. Published by AGEDI. Produced by GRID-Arendal, A Centre Collaborating with UNEP, Norway
Aizawa M, Asaoka K, Atsumi M, Sakou T (2007) Seaweed bioethanol production: the ocean sunrise project. Available from: http://ieeexplore.ieee.org/stamp/stamp.jsp?tp=andarnumber=4449162 Accessed 28 Mar 2015
Andersen KH, Mork M, Nilsen JEO (1996) Measurement of the velocity-profile in and above a forest of Laminaria hyperborea. Sarsia 81:193–196
Arenas F, Vas-Pinto F (2014) Marine algae as carbon sinks and allies to combat global warming. In: Pereira L, Neto JM (eds) Marine algae: biodiversity, taxonomy, environmental assessment and biotechnology. CRC Press, Boca Raton, pp. 178–193
Azam F, Fenchel T, Field JG, Gray JS, Meyerrell LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Bach QV, Sillero MV, Tran KQ, Skjermo J (2014) Fast hydrothermal liquefaction of a Norwegian macroalga: screening test. Algal Res 6(B):271–276
Barratt-Boyes M (2012) Pest may prove a source of plenty. New Zealand Aquaculture 46:8–9
Baruah K, Norouzitallab P, Sorgeloos P (2006) Seaweed: an ideal component for wastewater treatment for use in aquaculture. Aquaculture Europe 31:3–6
Bharathiraja B, Chakravarthy M, Kumar RR, Yogendran D, Yuvaraj D, Jayamuthunagai J, Kumar RP, Palani S (2015) Aquatic biomass (algae) as a future feed stock for bio-refineries: a review on cultivation, processing and products. Renew Sust Energ Rev 47:634–653
Bird MI, Wurster CM, de Paula Silva PH, Bass AM, de Nys R (2011) Algal biochar-production and properties. Bioresource Technol 102:1886–1891
Blaber SJM (2000) Tropical estuarine fishes: ecology, exploitation and conservation. Blackwell, Oxford
Bouillon S, Dahdouh-Guebas F, Rao AVVS, Koedam N, Dehairs F (2003) Sources of organic carbon in mangrove sediments: variability and possible ecological implications. Hydrobiologia 495:33–39
Bouillon S, Connoly RM (2009) Carbon exchange among tropical coastal ecosystems. In: Nagelkerken I (ed) Ecological connectivity among tropical ecosystems. Springer, Dordrecht, pp. 45–70
Cesar HJS, Ohman MC, Espeut B, Honkanen M (2000) An economic valuation of Portland Bright, Jamaica: an integrated terrestrial and marine protected area. Working paper 00/03, Institute for Environmental Studies, Free University, Amsterdam
Choi JH, Woo HC, Suh DJ (2014) Pyrolysis of seaweeds for bio-oil and bio-char production. Chem Eng Trans 37:121–126
Chopin T, Yarish C, Wilkes R, Belyea E, Lu S, Mathieson A (1999) A developing Porphyra/salmon integrated aquaculture for bioremediation and diversification of the aquaculture industry. J Appl Phycol 11:463–472
Christie H, Norderhaug KM, Fredriksen S (2009) Macrophytes as habitat for fauna. Mar Ecol Prog Ser 396:221–233
Chmura GL, Anisfield SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetlands soil. Glob Biogeochem Cycles 1111. doi:10.1029/2002GB001917
Chung IK (2015) Outlook for Korean aquaculture—seaweed aquaculture in Korea. AquaInfo magazine (English ed) 3:42–60
Chung IK, Beardall J, Mehta S, Sahoo D, Stojkovic S (2011) Using marine macroalgae for carbon sequestration: a critical appraisal. J Appl Phycol 23:877–886
Chung IK, Oak JH, Lee JA, Shin JA, Kim JG, Park KS (2013) Installing kelp forest/seaweed beds for mitigation and adaptation against global warming: Korean project overview. ICES J Mar Sci. doi:10.1093/icesjms/fss206
Cole AJ, Mata L, Paul NA, de Nys R (2014) Using CO2 to enhance carbon capture and biomass applications of freshwater macroalgae. GCB Bioenergy 6:637–645
Cramton P, Ockenfels A, Stoft S (2015) An international carbon-price commitment promotes cooperation. Econ Energy Environ Policy 4:51–64
Crawley KR, Hyndes GA, Vanderklift MA, Revill AT, Nichols PD (2009) Allochthonous brown algae are the primary food source for consumers in a temperate, coastal environment. Mar Ecol Prog Ser 376:33–44
Delille B, Borges AV, Delille D (2009) Influence of giant kelp beds (Macrocystis pyrifera) on diel cycles of pCO2 and DIC in the sub-Antarctic coastal area. Estuar Coast Shelf Sci 81:114–122
Dierssen HM, Zimmerman RC, Drake RA (2009) Burdige DJ (2009) potential export of unattached benthic macroalgae to the deep sea through wind-driven Langmuir circulation. Geophys Res Lett 36(4):L04602. doi:10.1029/2008GL036188
Duarte CM, Middelburg JJ, Caraco N (2005) Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2:1–8
Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N (2013) The role of coastal plant communities for climate change mitigation and adaptation. Nat Clim Chang 3:961–968
Eklöf JS, de la Torre-Castro M, Nilsson C, Rönnbäck P (2006) How do seaweed farms influence local fishery catches in a seagrass-dominated setting in Chwaka Bay. Zanzibar Aquat Living Resour 19:137–147
Eklöf JS, Msuya FE, Lyimo TJ, Buriyo AS (2012) Seaweed farming in Chwaka Bay: a sustainable alternative in aquaculture? In: de la Torre-Castro M, Lyimo TJ (eds) People, nature and research in Chwaka Bay. WIOMSA, Zanzibar, Tanzania, pp. 213–233
Emerton L, Kekulandala LDCB (2003) Assessment of economic value of Muthurajawela wetland. Occ. Pap. IUCN Sri Lanka (4): 28p. http://data.iucn.org/dbtw-wpd/edocs/2003-005.pdf
EPA (2014) Climate change indicators in the United States: Global greenhouse gas emissions. www.epa.gov/climatechange/indicators. Accessed 20 Mar 2016.
Fankhauser S, Tol RSJ (1996) Recent advancements in the economic assessment of climate change costs. Energ Policy 24:665–667
FAO (2014) The state of world fisheries and aquaculture 2012. Rome, 223 p
FAO (2016) FIGIS. Global aquaculture production 1950–2012. Food and Agriculture. Available from: http://www.fao.org/figis/servlet/TabSelector. Accessed 28 Mar 2016
Farrelly DJ, Everard CD, Fagan CC, McDonnell KP (2013) Carbon sequestration and the role of biological carbon mitigation: a review. Renew Sust Energ Rev 21:712–727
Fei XG (2004) Solving the coastal eutrophication problem by large scale seaweed cultivation. Hydrobiologia 512:145–151
Fourqurean JW, Duarte CM, Kennedy H, Marba N, Holmer M, Mateo MA, Apostolaki ET, Kendrick GA, Krause-Jensen D, McGlathery K, Serrano O (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:505–509
Fraser CI, Nikula R, Waters JM (2011) Oceanic rafting by coastal community. Proc R Soc B 278:649–655
Gao K, McKinley KR (1994) Use of macroalgae for marine biomass production and CO2 remediation: a review. J Appl Phycol 6:45–60
Gao K, Zheng Y (2010) Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessile (Rhodophyta). Glob Chang Biol 16:2388–2398
Gevaert F, Janguin MA, Davoult D (2008) Biometrics in Laminaria digitata: a useful tool to assess biomass, carbon and nitrogen contents. J Sea Res 60:215–219
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Harrold C, Light K, Lisin S (1998) Organic enrichment of submarine-canyon and continental shelf benthic communities by macroalgal drift imported form nearshore kelp forests. Limnol Oceanogr 43:669–678
He P, Xu S, Zhang H, Wen S, Day Y, Lin S, Yarish C (2008) Bioremediation efficiency in the removal of dissolved inorganic nutrients by the red seaweed, Porphyra yezoensis, cultivated in the open sea. Water Res 42:1281–1289
Hill R, Bellgrove A, Macreadie PI, Petrou K, Beardall J, Steven A, Ralph PJ (2015) Can macroalgae contribute to blue carbon? An Australian perspective. Limnol Oceanogr 60:1689–1706
Hobday AJ (2000) Abundance and dispersal of drifting kelp Macrocystis pyrifera rafts in the Southern California Bight. Mar Ecol Prog Ser 195:101–116
Hong DD, Hien MH, Son PN (2007) Seaweed from Vietnam used for functional food, medicine and biofertilizer. J Appl Phycol 19:817–826
Howard J, Hoyt J, Isensee K, Pidgeon E, Telszewski M (2014) Coastal blue carbon: methods for Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International union for conservation of nature factors in mangroves, tidal salt marshes, and seagrasses meadows. Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International union for conservation of nature. Arlington, Virginia, USA
Huo YZ, Wu HL, Chai ZY, Xu SN, Han F, Dong L, He PM (2012) Bioremediation efficiency of Gracilaria verrucosa for an integrated multi-trophic aquaculture system with Pseudosciaena crocea in Xiangshan harbor, China. Aquaculture 326-329:99–105
Hughes AD, Black KD, Campbell I, Davidson K, Kelly MS (2012) Does seaweed offer a solution for bioenergy with biological carbon capture and storage? Greenhouse Gas Sci Technol 2:402–407
Hyndes GA, Lavery PS, Doropoulos C (2012) Dual processes for cross boundaries subsidies: incorporation of nutrients from reef-derived kelp into a seagrass ecosystem. Mar Ecol Prog Ser 445:97–107
Hyndes GA, Nagelkerken I, McLeod RJ, Connolly RM, Lavery PS, Vanderklift MA (2014) Mechanisms and ecological role of carbon transfer within coastal seascapes. Biol Rev 89:232–254
IPCC (2014) Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri RK, Meyer LA (eds)] IPCC, Geneva, Switzerland, 151 pp
Ito Y, Nakano Y, Matsushita S, Mikami N, Yokoyama J, Kirihara S, Notoya M (2009) Estimations of quantities of carbon storage by seaweed and seagrass beds. Japan Fish Eng 46:135–146
Jotzo F (2012) Australia’s carbon price. Nat Clim Chang 2:475–476
Jackson GA (1984) Internal wave attenuation by coastal kelp stands. J Phys Oceanogr 14:1300–1306
Jansson C, Wullschleger SD, Kalluri UC, Tuskan GA (2010) Photosequestration: carbon biosequestration by plants and the prospects of genetic engineering. Bioscience 60:685–696
Johnson DL, Richardson PL (1977) On the wind-induced sinking of Sargassum. J Exp Mar Biol Ecol 28:255–267
Kaur CR, Ang M (2009) Seaweed culture and utilization in Malaysia status, challenges and economic potential. Seminar on developing the seaweed aquaculture sector in Malaysia, (Maritime Institute in Malaysia). Presented at the Seminar on developing the seaweed aquaculture sector in Malaysia, MIMA (Maritime Institute in Malaysia), Malaysia. 27 October 2009. www:mima.gov.my
Kim SS, Ly HV, Kim J, Choi JH, Woo HC (2013) Thermogravimetric characteristics and pyrolysis kinetics of alga Sargassum sp. biomass. Bioresour Technol 139:242–248
Kneib RT (1997) The role of tidal marshes in the ecology of estuarine nekton. Oceanogr Mar Biol Ann Rev 35:163–220
Komatsu T, Matsunaga D, Mikami A, Sagawa T, Boisnier E, Tatsukawa K, Aoki M, Ajisaka T, Uwai S, Tanaka K, Ishida K, Tanoue H, Sugimoto T (2008) Abundance of drifting seaweeds in eastern East China Sea. J Appl Phycol 20:801–809
Littler MM, Murray SN (1974) The primary productivity of marine macrophytes from a rocky intertidal community. Mar Biol 27:131–135
Lovas SM, Totum A (2001) Effect of the kelp Laminaria hyperborean upon sand dune erosion and water particle velocities. Coast Eng 44:37–63
Luisetti T, Jackson EL, Turner RK (2013) Valuing the European ‘coastal blue carbon’storage benefit. Mar Pollut Bull 71:101–106
MacKay D, Cramton P, Ockenfels A, Stoft S (2015) Price carbon—I will if you will. Nature 526:315–316
Mann KH (1972) Ecological energetics of the sea-weed zone in a marine bay on the Atlantic coast of Canada. II. Productivity of the seaweeds. Mar Biol 14:199–209
Maie N, Jaffe R, Miyoshi T, Childers DL (2006) Quantitative and qualitative aspects of dissolved organic carbon leached from senescent plants in an oligotrophic wetland. Biogeochemistry 78:285–314
Manley B (2016) Afforestation responses to carbon price changes and market certainties. Report for the ministry for primary industries. http://www.mfe.govt.nz/sites/default/files/media/Climate%20Change/Afforestation%20responses
Mcleod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
McHugh DJ (2003) A guide to seaweed industry. Food and Agricultural Organization, Rome, 106p
McKenzie PF, Bellgrove A (2009) Dislodgment and attachment strength of the intertidal macroalga Hormosira banksii (Fucales, Phaeophyceae). Phycologia 48:335–343
McVey JP, Stickney R, Yarish C, Chopin T (2002) Aquatic poly-culture and balanced ecosystem management: new paradigms for seafood production. In: Stickney RR, McVey JP (eds) Responsible aquaculture. CAB International, Oxford, pp. 91–104
MMAF (Ministry of Marine Affair and Fisheries Indonesia) (2014) Available from: www.kkp.go.id. Accessed 10 Oct 2016
MISA (Marine Innovation South Australia) (2011) Seaweed farming coming soon. MISA Snapshot issue 1. Available from: http://repository.seafdec.org.ph. Accessed 4 Oct 2014
Mitra A, Zaman S, Pramanick P, Bhattacharyya SB, Raha AK (2014) Stored carbon in dominant seaweeds of Indian Sundarbans. Pertanika J Trop Agric Sci 37:263–274
Mitra A, Zaman S (2014) Carbon sequestration by coastal floral community: a ground zero observation on blue carbon. TERI, New Delhi 428p
Muraoka D (2004) Seaweed resources as a source of carbon fixation. Bull Fish Res Agen 1:59–63
Murray BC, Pendleton L, Jenkins WA, Sifleet S (2011) Green payment for blue carbon: economic incentives for protecting threatened coastal habitats. Nicholas Institute for Environment Policy Solutions Report. Durham, North Carolina, USA, p 42
Nellemann C, Corcoran E, Duarte CM, Valdes L, De Young C, Fonseca L, Grimsditch G (2009) Blue carbon. A rapid response assessment. GRID-Arendal: United Nations Environment Programme
Nkemka VN, Murto M (2010) Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J Environ Manag 91:1573–1579
Notoya M (2011) Production of biofuel by macroalgae with preservation of marine resources and environment. In: Israel A, Einav R, Seckbach J (eds) Seaweed and their role in globally changing environments. Springer, New York, pp. 219–228
N’Yeurt A, Chynoweth D, Capron ME, Stewart J, Hasan M (2012) Negative carbon via ocean afforestation. Process Safe Environ Protect 90:467–474
Okuda K (2008) Coastal environment and seaweed-bed ecology in Japan. Kuroshio Science 2-1:15–20
Olafsson E, Johnstone RW, Ndaro SGM (1995) Effects of intensive seaweed farming on the meiobenthos in a tropical lagoon. J Exp Mar Biol Ecol 191:101–117
Olivier JG, Janssens-Maenhout G, Muntean M, Peters JAHW (2015) Trends in global CO2 emissions; 2015 Report, The Hague: PBL Netherlands Environmental Assessment Agency. European Commission, Joint Research Centre, Ispra
Orr M, Zimmer M, Jelinski DE, Mews M (2005) Wrack deposition on different beach types: spatial and temporal variation in the pattern of subsidy. Ecology 86:1496–1507
Paddack MJ, Estes JA (2000) Kelp forest fish population in marine reserves and adjacent exploited areas of central California. Ecol Appl 10:855–870
Pendleton L, Donato DC, Murray BC, Crooks S, Jenkins WA, Sifleet S, Craft C, Fourqurean JW, Kauffman JB, Marba N, Megonigal P, Pidgeon E, Herr D, Gordon D, Baldera A (2012) Estimating global blue carbon emission from conversion and degradation of vegetated coastal ecosystems. PLoS One 7:e43542
Phang SM, Yeong HY, Lim PE, Adibi Rahiman MN, Gan KT (2010) Commercial varieties of Kappaphycus and Eucheuma in Malaysia. Malaysian J Sci 29:214–224
Ramus J (1992) Productivity of seaweeds. In: Falkowski PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Plenum Press, NY, pp. 239–255
Raven JA, Cockell CS, La Rocha CL (2008) The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Phil Trans Roy Soc B 363:2641–2650
Roberts DA, Paul NA, Dworjanyn SA, Bird MI, de Nys R (2015) Biochar commercially cultivated seaweed for soil amelioration. Scientific Report 5:9665. doi:10.1038/srep09665
Samonte-Tan G, Armedilla MC (2004) Economic valuation of Philippine coral reefs in the South China Sea biogeographic region. Nat Coral Reef Review Ser 3:1–39
Schlesinger WH (1997) Biogeochemistry: an analysis of global change, 2nd edn. Academic Press, San Diego
Semesi S, Beer S, Bjork M (2009) Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous algae in a tropical seagrass meadow. Mar Ecol Prog Ser 382:41–47
Siikamaki J, Sanchirico JN, Jardine S, McLaughlin D, Morris DF (2012) Blue carbon: global options for reducing emissions from the degradation and development of coastal ecosystems. Resource for the Future, Washington. 70p.
Smetacek V, Zingone A (2013) Green and golden seaweed tides on the rise. Nature 504:84–88
Smith SV (1981) Marine macrophytes as a global carbon sink. Science 211:838–840
Sondak CFA, Chung IK (2015) Potential blue carbon from coastal ecosystems in the Republic of Korea. Ocean Sci J 50:1–8
Suh DJ, Choi JH, Woo HC (2014) Pyrolysis of seaweeds for bio-oil and bio-char production. Chem Eng Trans 37:121–126
Tang Q, Zhang J, Fang J (2011) Shellfish and seaweed mariculture increase atmospheric CO2 absorption by coastal ecosystem. Mar Ecol Prog Ser 424:97–104
Thayer G, Bjorndal K, Ogden J, Williams S, Zieman J (1984) Role of larger herbivores in seagrass communities. Estuaries 7:351–376
Torres LG (2009) A kaleidoscope of mammal, bird and fish: habitat use patterns of top predators and their prey in Florida Bay. Mar Ecol Prog Ser 375:289–304
Trevathan-Tackett SM, Kelleway JJ, Macreadie PI, Beardall J, Ralph P, Bellgrove A (2015) Comparison of marine macrophytes for their contributions to blue carbon sequestration. Ecology 96:3043–3057
Turan G, Neori A (2011) Intensive seaweed aquaculture: a potent solution against global warming. In: Israel A, Einav R, Seckbach J (eds) Seaweed and their role in globally changing environments. Springer, Dordrecht, pp. 359–372
Valderrama D (2012) Social and economic dimensions of seaweed farming: a global review. 2012. IIFET, Tanzania Proceedings
Vasquez JA, Zuniga S, Tala F, Piaget N, Rondriquez DC, Alonso-Vega JM (2014) Economic valuation of kelp forests in northern Chile: values of goods and services of the ecosystem. J Appl Phycol 26:1081–1088
Vetter EW, Dayton PK (1998) Macrofaunal communities within and adjacent to a detritus-rich submarine canyon system. Deep-Sea Res II 45:25–54
Vierros M (2013) Communities and blue carbon: the role of traditional management systems in providing benefits for carbon storage, biodiversity conservation and livelihoods. Climate Change. doi:10.1007/s10584-013-920-3
Walsh M, Watson L (2011) A market analysis towards the further development of seaweed aquaculture in Ireland. Irish Sea Fisheries Board, Dublin
Wattage P (2011) Valuation of ecosystem services in coastal ecosystems: Asian and European perspectives. Ecosystem Services Economics (ESE). Working Paper Series No. 8. The United Nations Environment Program (UNEP) Publication Series
Wernberg T, Vanderklift MA, How J, Lavery PS (2006) Export of detached macroalgae from reefs to adjacent seagrass beds. Oecologia 147:692–701
Widowati T, Pramono GH, Rusmanto A, Munajati SL (2012) Spatial analysis: the effectiveness of seaweed as a catalyst for improving ecologic and economic qualities in Takalar water area South Sulawesi. Proceedings of Global Geospatial Conference 2012 Québec City, Canada, 14–17 May 2012
Wolanski E (1992) Hydrodynamics of mangrove swamps and their coastal waters. Hydrobiologia 247:141–161
Xu M, Sakamoto S, Komatsu T (2016) Attachment strength of the subtidal seaweed Sargassum horneri (Turner) C. Agardh varies among development stages and depths. J Appl Phycol. doi:10.1007/s10811-016-0869-5DOI
Yanagisawa M, Kawai S, Murata K (2013) Strategies for the production of high concentrations of bioethanol from seaweed: production of high concentrations of bioethanol from seaweed. Bioengineered 4:224–235
Yang YF, Fei XG, Song JM, Hu HY, Wang GC, Chung IK (2006) Growth of Gracilaria lemaneiformis under different cultivation conditions and its effects on nutrient removal in Chinese coastal waters. Aquaculture 254:248–255
Zehetner F (2010) Does organic carbon sequestration in volcanic soils offset volcanic CO2 emissions? Quaternary Sci Rev 29:1313–1316
Zemke-White WL, Ohno M (1999) World seaweed utilization: an end-of-century summary. J Appl Phycol 11:369–376
Zemke-White WL, Smith JE (2006) Environmental impacts of seaweed farming in the tropics. In: Critchley AT, Ohno M, Largo D (eds) Seaweed resources. Expert Center for Taxonomic Identification (ETI), Univ. Amsterdam. CD-ROM World Seaweed Resources—. Version, 1
Acknowledgements
This work has been supported by the National Research Foundation of Korea, Marine Research Institute, Pusan National University (NRF-2013R1A1A2009359), and DIKTI Scholarship from the Indonesia Ministry of National Education and Culture for CFAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s10811-017-1147-x.
Rights and permissions
About this article
Cite this article
Sondak, C.F.A., Ang, P.O., Beardall, J. et al. Carbon dioxide mitigation potential of seaweed aquaculture beds (SABs). J Appl Phycol 29, 2363–2373 (2017). https://doi.org/10.1007/s10811-016-1022-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-1022-1