Abstract
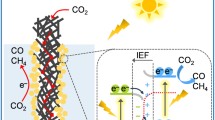
How to overcome the bottleneck of Pt–based catalysts is a research focus in the field of energy conversion, and the combination of metal oxide with Pt–based catalysts is a promising strategy to avoid platinum aggregation and introduce novel photosensitivity into traditional Pt catalysts by virtue of the photosensitivity of metal oxide semiconductors. In this work, Pt–MoOx/graphene (S) (simplified as Pt–MoOx/GN (S)) was synthesized by a solid–phase reaction with Na2MoO4 as the Mo precursor, in which Na2MoO4 was partially reduced to MoO2, leading to the formation of MoOx in the composite. The integration of MoOx with Pt results in the uniform dispersion of Pt nanoparticles. Electrochemical tests reveal that the catalytic activity of Pt–MoOx/GN (S) is comparable to that of commercial 30% PtRu/C–JM toward the oxygen reduction reaction via a 4–electron transfer process. In particular, Pt–MoOx/GN (S) displays a more positive reduction potential and stronger stability, which is ascribed to the synergies between MoOx and Pt. Owing to the introduction of photo–responsive MoOx species, Pt–MoOx/GN (S) also exhibits significantly higher mass activity under simulated sunlight illumination, which is 1.62 times as much as that without illumination. The result is in favor of converting solar energy into electric energy during a traditional electrocatalytic process.
Graphic abstract








Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gewirth AA, Thorum MS (2010) Electroreduction of dioxygen for fuel-cell applications: materials and challenges. Inorg Chem 41:3557–3566. https://doi.org/10.1021/ic9022486
Mao J, Chen W, He D, Wan J, Pei J, Dong J, Wang Y, An P, Jin Z, Xing W (2017) Design of ultrathin Pt-Mo-Ni nanowire catalysts for ethanol electrooxidation. Sci Adv 3:e1603068. https://doi.org/10.1126/sciadv.1603068
Han C, Jing W, Gong Y, Xuan X, Li H, Yong W (2013) Nitrogen-doped hollow carbon hemispheres as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline medium. J Mater Chem A 2:605–609. https://doi.org/10.1039/c3ta13757k
Gang W, Piotr Z (2013) Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc Chem Res 46:1878–1889. https://doi.org/10.1021/ar400011z
He LL, Zheng JN, Pei S, Zhong SX, Wang AJ, Chen Z, Feng JJ (2015) Facile synthesis of platinum–gold alloyed string-bead nanochain networks with the assistance of allantoin and their enhanced electrocatalytic performance for oxygen reduction and methanol oxidation reactions. J Power Sources 276:357–364. https://doi.org/10.1016/j.jpowsour.2014.11.119
Wang B (2005) Recent development of non-platinum catalysts for oxygen reduction reaction. J Power Sources 152:1–15. https://doi.org/10.1016/j.jpowsour.2005.05.098
Sun T, Wu Q, Che R, Bu Y, Jiang Y, Li Y, Yang L, Wang X, Hu Z (2015) Alloyed Co–Mo nitride as high-performance electrocatalystfor oxygen reduction in acidic medium. ACS Catal 5:1857–1862. https://doi.org/10.1021/cs502029h
Min P, Li C, Ling D, Jian Z, Su D, Li W, Liang C (2010) Microwave-assisted preparation of Mo2C/CNTs nanocomposites as efficient electrocatalyst supports for oxygen reduction reaction. Ind Eng Chem Res 175:275–278. https://doi.org/10.1021/ie901741c
da Silva GC, Fernandes MR, Ticianelli EA (2018) Activity and stability of Pt/IrO2 bifunctional materials as catalysts for the oxygen evolution/reduction reactions. ACS Catal 8:2081–2092. https://doi.org/10.1021/acscatal.7b03429
Gao W, Zhang Z, Dou M, Wang F (2019) Highly dispersed and crystalline Ta2O5 anchored Pt electrocatalyst with improved activity and durability towards oxygen reduction: promotion by atomic-scale Pt–Ta2O5 interactions. ACS Catal 9:3278–3288. https://doi.org/10.1021/acscatal.8b04505
Wang ZB, Yin GP, Lin YG (2007) Synthesis and characterization of PtRuMo/C nanoparticle electrocatalyst for direct ethanol fuel cell. J Power Sources 170:242–250. https://doi.org/10.1016/j.jpowsour.2007.03.078
Imran S, Muhammad S, Dae Joon K (2010) MoO3 and Cu0.33MoO3 nanorods for unprecedented UV/Visible light photocatalysis. Chem Commun 46:4324–4326. https://doi.org/10.1039/c000003e
Miyauchi M, Nakajima A, Watanabe T, Hashimoto K (2002) Photocatalysis and photoinduced hydrophilicity of various metal oxide thin films. Chem Mater 14:2812–2816. https://doi.org/10.1021/cm020076p
Bakos I, Borbáth I, Pászti Z, Tompos A (2018) Design and investigation of molybdenum modified platinum surfaces for modeling of CO tolerant electrocatalysts. Top Catal 61:1385–1395. https://doi.org/10.1007/s11244-018-1035-x
Yu S, Liu Q, Yang W, Han K, Wang Z, Hong Z (2013) Graphene-CeO2 hybrid support for Pt nanoparticles as potential electrocatalyst for direct methanol fuel cells. Electrochim Acta 94:245–251. https://doi.org/10.1016/j.electacta.2013.01.149
Zhu J, Wang D, Lin W, Lang X, You W (2013) Facile synthesis of sulfur coated SnO2–graphene nanocomposites for enhanced lithium ion storage. Electrochim Acta 91:323–329. https://doi.org/10.1016/j.electacta.2012.12.116
Suryamas AB, Anilkumar GM, Sago S, Ogi T, Okuyama K (2013) Electrospun Pt/SnO2 nanofibers as an excellent electrocatalysts for hydrogen oxidation reaction with ORR-blocking characteristic. Catal Commun 33:11–14. https://doi.org/10.1016/j.catcom.2012.12.014
Kakaei K, Rahimi A, Husseindoost S, Hamidi M, Javan H, Balavandi A (2016) Fabrication of Pt–CeO2 nanoparticles supported sulfonated reduced graphene oxide as an efficient electrocatalyst for ethanol oxidation. Int J Hydrogen Energy 41:3861–3869. https://doi.org/10.1016/j.ijhydene.2016.01.013
Hwang J, Yoon D, Kweon B, Chang W, Kim J (2016) A simple, one-pot synthesis of molybdenum oxide-reduced graphene oxide composites in supercritical methanol and their electrochemical performance. RSC Adv 6:108298–108309
Guha P, Mohanty B, Thapa R, Kadam RM, Jena BK (2020) Defects engineered MoO2 nanostructures as an efficient electrocatalyst for oxygen evolution reaction. ACS Appl Energy Mater 3:5208–5218. https://doi.org/10.1021/acsaem.9b02551
Zhou X, Zhao X, Xie F, Jin Z, Tang Z (2020) Plasmonic hybrid Mo/MoO2 nanospheres as surface-enhanced raman scattering substrates for molecular detection. ACS Appl Nano Mater 3:5656–5664. https://doi.org/10.1021/acsnm.0c00883
Xia C, Zhou Y, Velusamy DB, Farah AA, Li P, Jiang Q, Odeh IN, Wang Z, Zhang X, Alshareef HN (2018) Anomalous Li storage capability in atomically thin two-dimensional sheets of nonlayered MoO2. Nano Lett 18:1506–1515. https://doi.org/10.1021/acs.nanolett.7b05298
Li J, Ye Y, Ye L, Su FY, Ma Z, Huang J, Xie H, Doronkin DE, Zimina A, Grunwaldt JD (2019) Sunlight induced photo-thermal synergistic catalytic CO2 conversion via localized surface plasmon resonance of MoO3-x. J Mater Chem A 7:2821–2830. https://doi.org/10.1039/c8ta10922b
Xu K, Duan S, Tang Q, Zhu Q, Yuan C (2019) P–N heterointerface-determined acetone sensing characteristics of α-MoO3@NiO core@shell nanobelts. CrystEngComm 21:5834–5844. https://doi.org/10.1039/c9ce00742c
Li Z, Xu S, Xie Y, Wang Y, Lin S (2018) Promotional effects of trace Bi on its highly catalytic activity for methanol oxidation of hollow Pt/graphene catalyst. Electrochim Acta 264:53–60. https://doi.org/10.1016/j.electacta.2018.01.096
Gao H, Liao S, Zeng J, Xie Y (2011) Platinum decorated Ru/C: effects of decorated platinum on catalyst structure and performance for the methanol oxidation reaction. J Power Sources 196:54–61. https://doi.org/10.1016/j.jpowsour.2010.07.040
Suresh C, Mutyala S, Mathiyarasu J (2016) Support interactive synthesis of nanostructured MoS2 electrocatalyst for oxygen reduction reaction. Mater Lett 164:417–420. https://doi.org/10.1016/j.matlet.2015.11.052
Lee K-H, Lee Y-W, Kwak D-H, Moon J-S, Park A-R, Hwang E-T, Park K-W (2014) Single-crystalline mesoporous Mo2N nanobelts with an enhanced electrocatalytic activity for oxygen reduction reaction. Mater Lett 124:231–234. https://doi.org/10.1016/j.matlet.2014.03.097
Dey A, Nayak MK, Pradeepkumar MS, Porwal D, Esther ACM (2017) Studies on Mo doped vanadium oxide film by pulsed RF magnetron sputtering: Mo doped vanadium oxide film. Surf Interface Anal 49:805–808. https://doi.org/10.1002/sia.6226
Park JY, Kim S (2013) Preparation and electroactivity of polymer-functionalized graphene oxide-supported platinum nanoparticles catalysts. Int J Hydrogen Energy 38:6275–6282. https://doi.org/10.1016/j.ijhydene.2012.12.059
Yalan C, Jingtong Z, Peng G, Haijun L, Zhaojie W, Ming L, Tian Z, Shutao W, Yan Z, Xiaoqing L (2018) Coupled heterostructure of Mo-Fe selenide nanosheets supported on carbon paper as an integrated electrocatalyst for efficient hydrogen evolution. ACS Appl Mater Interfaces 10:27787–27794. https://doi.org/10.1021/acsami.8b08007
Khyzhun OY, Bekenev VL, Solonin YM (2008) Electronic structure of face-centred cubic MoO2: a comparative study by the full potential linearized augmented plane wave method, X-ray emission spectroscopy and X-ray photoelectron spectroscopy. J Alloys Compd 459:22–28. https://doi.org/10.1016/j.jallcom.2007.04.281
Zhuang L, Li Q, Chen S, Hou X, Lin J (2014) In-situ preparation of porous carbon-supported molybdenum dioxide and its performance in the oxidative desulfurization of thiophene. J Mater Sci 49:5606–5616. https://doi.org/10.1007/s10853-014-8273-5
Curry RA, Sparks D, Sande JVD (2011) Facile construction of manganese oxide doped carbon nanotube catalysts with high activity for oxygen reduction reaction and investigations into the origin of their activity enhancement. ACS Appl Mater Interfaces 3:2601–2606. https://doi.org/10.1021/am200426q
Raana KS, Sajad Y, Tran DH, Zhao C, Thompson PM (2018) Role of oxygen vacancy defects in the electrocatalytic activity of substoichiometric molybdenum oxide. J Phys Chem C 122:18212–18222. https://doi.org/10.1021/acs.jpcc.8b03536
Li Z, Ye L, Lei F, Wang Y, Xu S, Shen L (2016) Enhanced electro-photo synergistic catalysis of Pt (Pd)/ZnO/graphene composite for methanol oxidation under visible light irradiation. Electrochim Acta 188:450–460. https://doi.org/10.1016/j.electacta.2015.11.149
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21571034) and the Research Foundation of the Education Department of Fujian Province (Grant No. JAT170150).
Author information
Authors and Affiliations
Contributions
Experimental investigation: XD, YW, YL. Data curation, Software, Formal analysis, Visualization and Writing of the Original Draft: XD, YW. Validation: YL. Conceptualization, Methodology, Resources, Funding acquisition, Project administration, Supervision, and writing–review & editing: ZL, SL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors of the manuscript have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, X., Wu, Y., Lai, Y. et al. Synthesis of Pt–MoOx/graphene composite and its electro–photo synergistic catalysis for oxygen reduction reaction. J Appl Electrochem 52, 115–123 (2022). https://doi.org/10.1007/s10800-021-01622-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01622-5