Abstract
We investigate the capability of information from electronic health records of an emergency department (ED) to predict patient disposition decisions for reducing “boarding” delays through the proactive initiation of admission processes (e.g., inpatient bed requests, transport, etc.). We model the process of ED disposition decision prediction as a hierarchical multiclass classification while dealing with the progressive accrual of clinical information throughout the ED caregiving process. Multinomial logistic regression as well as machine learning models are built for carrying out the predictions. Utilizing results from just the first set of ED laboratory tests along with other prior information gathered for each patient (2.5 h ahead of the actual disposition decision on average), our model predicts disposition decisions with positive predictive values of 55.4%, 45.1%, 56.9%, and 47.5%, while controlling false positive rates (1.4%, 1.0%, 4.3%, and 1.4%), with AUC values of 0.97, 0.95, 0.89, and 0.84 for the four admission (minor) classes, i.e., intensive care unit (3.6% of the testing samples), telemetry unit (2.2%), general practice unit (11.9%), and observation unit (6.6%) classes, respectively. Moreover, patients destined to intensive care unit present a more drastic increment in prediction quality at triage than others. Disposition decision classification models can provide more actionable information than a binary admission vs. discharge prediction model for the proactive initiation of admission processes for ED patients. Observing the distinct trajectories of information accrual and prediction quality evolvement for ED patients destined to different types of units, proactive coordination strategies should be tailored accordingly for each destination unit.
Similar content being viewed by others
1 Introduction and motivation
Overcrowding has long been identified as a critical issue in emergency department (ED) management [1,2,3,4]. In response, various approaches have been suggested including the adoption of fast-track units, advanced patient triage strategy, and the implementation of Six Sigma and “lean” programs to alleviate crowding in the ED [5,6,7,8,9,10,11]. However, prolonged patient “boarding” delays (i.e., delays experienced by patients admitted into the hospital by being held up in the ED due to admission, bed coordination, and transport delays), is known to be one of the most significant factors contributing to ED crowding [2, 12]. In particular, Pines et al. have investigated hospitals in 15 different countries and identified that the common main cause for ED crowding is the boarding of admitted ED patients [13, 14]. There is also a growing body of literature reporting the gravity of ED patient boarding in different countries and attesting to its negative clinical, operational, and financial impacts on healthcare management [15,16,17,18,19].
To improve patient flow out of the ED, different approaches have been introduced in the field of healthcare management science and operations research/management. Saghafian et al. [20] studied a patient streaming strategy that separates ED patients into two different streams according to anticipated admission decisions and discussed that the strategy could help alleviate the ED patient boarding issue. Shi et al. [21] investigated the inpatient unit (IU) discharge process and suggested that ED boarding delays can be reduced by altering inpatient discharge times. Osorio and Bierlaire [22] analyzed finite capacity queues to identify sources of bed blocking and suggest operational implications. Among others, as a potential remedy to considerably reduce boarding delay, the ideas of “early task initiation” (e.g., proactively checking admission criteria and seeking admission approval from hospitals) or “proactive resource preparation” for admission (e.g., preparing an inpatient bed for an impending admission) have received increasing academic and industrial attention [23,24,25,26]. The underlying premise is that modern-day electronic health record systems can support the realization of real-time hospital admission prediction models while the patient is still undergoing ED treatment. The rationale behind this proactive strategy for patient flow coordination is that bed request and preparation to admit a patient are often delayed until admission is certain [23]. When there are a large number of patient discharges at IUs combined with high demand for clean IU beds for admissions, bed management becomes a prioritization problem where environmental service staff (responsible for cleaning and turning around beds) should be dispatched according to admissions coming into each IU. This is symptomatic of most EDs for IU discharges tend to surge around midday (after morning rounds by providers) and environmental services struggle to keep up with the rate of patient discharge and are forced to clean beds based on admission priority lists rather than turning around beds as they become vacated. The patterns in Fig. 1 illustrate this situation routinely occurring within the ED-to-IU workflow of a leading level-1 trauma healthcare facility in the United States Midwest, where this study is conducted (HEM/ONC/BMP stands for hematology/oncology/blood and marrow transplant unit.). The ED suffers from severe crowding (indicated by the dashed line with the secondary y-axis) influenced by the increasing levels of patient boarding (indicated by the solid line with the primary y-axis) in the afternoon and evening (Fig. 1a). Even though there are unoccupied inpatient beds during the same period (Fig. 1b) generated by the high rate of IU patient discharges in the afternoon (Fig. 1c), less effective (i.e., reactive) bed management leads to excessive boarding delays.Footnote 1 It is under these circumstances that ED patient admission predictions combined with proactive coordination can limit the trajectory of boarding delay by judiciously allocating resources and proactively initiating tasks for predicted admissions [27,28,29,30].
ED and IUs patient flow graphs. Note: The shaded area indicates the period within a day when the ED suffers from severe crowding. During this period, there are unoccupied inpatient beds for most weekdays that could help alleviate the ED congestion issue when related processes are proactively coordinated
While there has been some progress with ED patient admission prediction modeling research to enable proactive coordination [23, 26, 31,32,33,34,35,36,37], the models are lacking in their granularity to allow operationalization of prediction outcomes in real-world settings. Just predicting that a patient will be “admitted” will not necessarily allow full proactive coordination of resources across the ED-to-IU workflow for streamlined patient admission and flow since in most cases the allocation of necessary resources, e.g., inpatient beds, requires information on “which IU the patient is likely to be admitted”. Generally, inpatient care can be categorized into three main types based on the intensity of required care [38]: general care (least intensive care), telemetry/stepdown care (intermediate care), and intensive care. They in turn define the three main types of IUs, i.e., general practice unit (GPU), telemetry unit (TU; also known as stepdown unit), and intensive care unit (ICU). While GPU and ICU constitute traditional and well-established inpatient care units in hospitals, TU has been increasingly adopted in hospitals for providing an intermediate level of care for patients with requirements between that of GPU and ICU. The comparable settings can be readily found in the literature describing other healthcare institutes, where we observe that the inpatient care structure comprised of GPU, TU, and ICU is becoming common in many different countries [39,40,41].
The study hospital has the aforementioned three main types of IUs for regular IU admissions. While a disposition decision can further specify the most proper specialty unit within a main IU for a patient, if all beds in that specialty unit are in use, the patient is generally transferred to the second most proper specialty IU and so on (also termed “overflow”) and the disposition decision is updated accordingly. It is worth noting that the features of inpatient beds and accessories are generally common across a main unit, and this in turn constrains overflow to happen within the same main unit. In addition to the overflow policy, most hospitals physically separate ICU, TU, and GPU, pooling resources (e.g., beds and nursing services) within each main unit, exclusively. Therefore, given routine patient overflow occurring within a main IU and the resource pooling strategy at the study hospital, we seek to predict disposition decisions at the level of the main IUs. In addition, the ED physicians at the study hospital were only making disposition decisions at the main IU level about half the time (without further specifying any specialty) further justifying the prediction approach.
In addition to the IUs, the study hospital also operates an observation unit (OU) for providing extended care to patients who finished care processes in the ED. OUs are increasingly used as a short-stay (< 23 h) clinical decision unit for patients who require further observation and treatment. Even though the OU is not a part of regular IUs in most hospitals, it plays a significant role to control demand to IUs, and a significant number of ED patients can be sent to OU (around 6% in the study hospital).
The main goal of this research is to predict disposition decisions at the level of the main IUs and the OU for facilitating the unit-specific proactive coordination of admission processes to reduce ED patient boarding. Furthermore, our prediction modeling strategy incorporates the progressive nature of ED care processes, where more clinical information is revealed and accumulated for the patient as he/she goes through more ED processes (e.g., monitoring and testing) and treatment. This is the first study to conceptualize and evaluate the ED disposition decision prediction problem in the context of proactive coordination.
2 Methods
2.1 Study design
In this study, we define three distinct levels of classification schemes for modeling disposition decisions, considering both practical and academic relevance (Fig. 2b). At the first level (denoted C1), the outcome of ED patient disposition decision classification is the binary admission decision (i.e., admission vs. discharge). The OU class patients are included in the admission class.
The C1 classification scheme has been adopted in most of the ED disposition decision prediction modeling research to date [23, 26, 31,32,33,34,35,36,37]. Due to its relatively simple structure, it could produce the most accurate results. At the second level (C2), the admission class at the C1 classification scheme is further segmented into two subclasses, i.e., IU vs. OU admission. While the IU class is regarded as official admission, OU patients may not be considered as “admitted” depending on the hospital even though OU patients could have features that are clinically similar to IU patients. Rather, the OU treatment is often regarded as “extended ED care”, and the mechanism of patient transfers to OUs is different from regular IUs in many hospitals. Finally, at the most granular level (C3), the IU class at C2 is further categorized into three main IU classes, i.e., ICU, TU, and GPU. We believe that the C3 classification scheme is where the most significant operational benefit can be derived by enabling the unit-specific proactive coordination of admission processes across the ED-to-IU workflow. To provide further justification for the need to model each classification scheme, we summarize the possible clinical and operations management applications and use cases for prediction results stemming from each classification scheme in Table 1. In doing so, we considered general resource allocation practices in hospitals to provide a common and general description. Note that the table lists the incremental utility of prediction results at each classification scheme, i.e., utility of classification at C3 also includes the utility of C2 classification; C2 includes the utility of C1.
As discussed earlier, each IU class at C3 can be further subdivided into specialty subunits. For instance, in the study hospital, the GPU has 12 distinct subunits based on specialty of care, including general internal medicine, nephrology, obstetrics/gynecology, neurology, pulmonary, and so on. Besides the overflow policy, about half the time, ED physicians only make disposition decisions at the main IU level without further specifying any specialty. Therefore, in this study, we focus on predicting the disposition decision at the level of the five classes at C3, considering its higher practical relevance. As expected, as we increase the granularity of prediction (i.e., from C1 through C3), the prediction problem becomes more challenging. Considering how the classes at C3 (the ICU, TU, GPU, OU, and discharge classes) are defined, the classification model should be able to discriminate the “clinical care intensity” of ED patients, which makes the prediction task challenging. Moreover, since disposition decisions and clinical care decisions (e.g., ordering laboratory/imaging tests and other clinical interventions) will not be completely consistent across different physicians, it is important to check how effectively data-driven machine-based prediction models can match the actual disposition decisions by exploiting clinical, demographic, and operational data.
2.2 Study setting and population
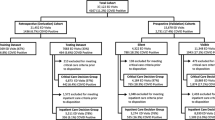
The study is based on electronic health record data collected at the ED of an academic urban level-1 trauma center, which delivers comprehensive care services including the fields of cardiology, cardiovascular surgery, neurology, neurosurgery, orthopedics and sports medicine, organ transplants, and treatments for prostate, breast and lung cancers. The data items were collected for the period from May 2014 to April 2016 and covers 184,895 patient visits. After accounting for abnormal departures (including patients leaving without completing services, transfers to other facilities, as well as patients who went to units that are not regarded as regular IUs, e.g., catheterization laboratory and perioperative unit), 172,809 patients remain in the dataset, which corresponds to 93.5% of the total visits (Fig. 2a). We provide general statistics around the study hospital and ED in Table 2 (based on information from the study data period). Because our dataset includes no patient-identifying information (PII data was removed by information technology staff before sharing the dataset), the study is exempted by the institutional review board of the study hospital.
2.3 Study protocol and methodology
We first introduce the proposed classification strategy briefly with few associated concepts from data science. The ED disposition prediction inherently presents a hierarchical, mandatory-leaf classification structure, where no two parent nodes share a common child node, and the most proper class is always found at the lowest level (Fig. 2b). The main approaches to tackle hierarchical classification problems can be classified into two main categories: “big-bang” and “top-down” [42]. While the top-down approach starts its classification task from the parent node and uses the obtained prediction outcomes for classification at its child nodes, the big-bang approach classifies the most proper class for the full problem with a single model. Given the hierarchical structure of ED disposition and the class membership imbalance at the C1 classification scheme (most patients, 75.3%, are negative [discharged] in the study hospital ED), the top-down approach would incur serious challenges at the downstream levels (C2 and C3). This is because false positive predictions occurring in the dominant negative class (discharge class in our case) at C1 propagate down the hierarchy and greatly affect the predictions for minor classes at C3. Especially, when the problem is of a mandatory-leaf node structure, false positive cases will spread throughout the hierarchical classification tree. Therefore, we choose to adopt the big-bang approach for predicting disposition decisions. Moreover, since each prediction class level could bring about their own operational benefits (as shown in Table 1), we built classification models at each level. Hence, we model and analyze the mandatory-leaf node, tree-structured ED disposition classification problem with the big-bang classifier per level approach.
In addition, we also consider the temporal aspects of ED disposition prediction. The diagnosis uncertainty decreases through the ED caregiving processes [26, 36]. Specifically, we identify four different ED caregiving epochs: “patient arrival at ED” (denoted T1-Arrival), “triage complete” (T2-Triage), “first provider encounter” (T3-Initial Assessment), and “first set of laboratory results returned” (T4-Initial Lab Results), which is graphically represented in Fig. 3. At T1-Arrival, ED patients arrive with some basic information such as arrival time/mode, prior ED visit history, health history (International Classification of Diseases, Tenth Revision, ICD-10 codes, from previous ED visits), and demographics. Then, patients go through triage processes, where the patient’s vital signs and chief complaints are recorded (T2-Triage). T2-Triage is when most admission decision prediction models have been built in the literature [31,32,33,34,35]. At T3-Initial Assessment, ED care providers examine patients, issue orders for laboratory/imaging tests and decide on other clinical interventions. We incorporate only the first set of laboratory/imaging test items ordered and other clinical interventions provided within 30 min of the patient’s first encounter with care providers, assuming that these items are dependent on examination at T3-Initial Assessment (upon examining the results from these tests, care providers can order additional tests and provide more clinical interventions downstream within the ED caregiving cycle, which are outside the scope of data employed for the T3-Initial Assessment setting). T4-Initial Lab Results indicates the time when the results for the first set of laboratory orders are fully reported. With the motivation of this study being “proactive” coordination within the ED-to-IU workflow, there is no point in making accurate disposition predictions using information that arrives too late in the ED caregiving cycle when the final disposition decision is already available or imminent. Unlike the laboratory tests, the results for radiology tests are not available electronically in a form that can be readily coded and are not included in this study. Considering the progressive nature of information accumulation throughout the ED caregiving process, the prediction model at T2-Triage incorporates predictors gained until epoch T2-Triage, the prediction model at T3-Initial Assessment incorporates predictors gained until epoch T3-Initial Assessment, and the prediction model at T4-Initial Lab Results incorporates predictors gained until epoch T4-Initial Lab Results.
Table 3 describes the time spans between these different ED care epochs in the study hospital over a two-year period (May 2014 to April 2016). Of note is the median “door to disposition decision” time of about four and a half hours for admitted ED patients and the median “disposition decision to admitted ED patient departure” (i.e., boarding) of about three hours for admitted patients.
While various classification modeling techniques that include multinomial logistic regression, multilayer perceptron neural networks, and support vector machines are applied, we do not see any notable difference in prediction performance that is worthy of detailed discussion in this study. Also, to enable rapid model calibration for individual hospitals and operationalization of the proposed early task initiation scheme, “simpler” and “explainable” models, such as multinomial logistic regression, should be favored over complex “black box” models (such as neural networks). Hence, we present in detail the results gained by a well-established approach, multinomial logistic regression in the Results section, and provide modeling hyperparameters tested in other machine learning models as well as their results (for the C3 classification scheme at T4-Initial Lab Results) in the Appendix (Table 11 and Table 12, respectively). Multinomial logistic regression is an extension of simple logistic regression for estimating the association between a set of predictors and a nominal outcome that has multiple categories (classes). In this study, we model logistic models that generalize the simple logistic regression by setting a baseline category among c categories (similar to the simple logistic regression that introduces a dummy category) with p number of predictors [43]. Therefore, we solve c − 1 logit equations having (c − 1) × p parameters as follows:
where j is the category index, j′ is the baseline category, αj is the intercept for category j, βj is a vector having p coefficients for predictors, and πij is the probability that the membership of ith observation is j. The probability can be calculated by rewriting Eq. 1 as follows:
The model finds parameter βj by maximizing the likelihood of the data. The entire dataset is split into two parts: first 85% of the patient visits for training the models (146,888 visits) and the rest for testing (25,921 visits). We only report prediction results obtained from the testing dataset in the Results section.
2.4 Predictor variable transformation and selection
To provide details about the predictors employed by the prediction models, Table 4 presents the information on how each set of predictors is transformed and entered the models (including the feature categorization rules from the Centers for Medicare and Medicaid Services [44] and the National Library of Medicine at the National Institute of Health [46]). All the feature selection and categorization methods are applied to the training dataset to extract the feature transformation rules, then the rules are applied to the testing data for preprocessing. In particular, the chi-square test is a fundamental and well-established approach that can measure the extent of dependence (or independence) among variables having multiple class levels [47]. The χ2 statistic of variable X is defined as follows:
where Oij is the observed frequency count for the ith level of the categorical variable X for class j, and Eij is the expected frequency count for the ith level of the categorical variable X for class j. If variables entail a high χ2 value, it infers that the variables deviate significantly from the independence assumption and regarded relevant to each other.
A chi-square statistic-based filter method is designed and applied as a feature selection mechanism in this study. In particular, to handle the predictors having excessively many categories, we conducted feature selection in two stages, i.e., within a predictor and between predictors. First, within-predictor feature selection is performed when a predictor has more than 50 categories (including patient arrival mode, chief complaint, and insurance plan predictors) that would unnecessarily increase computational complexity during the between-predictors feature selection process (the second stage). Then, in the second stage, supervised feature selection is performed among predictors obtained through the first stage selection, by building prediction models and fitting on the training dataset. This second stage gives the proper threshold of a chi-square statistic that optimally filters in and out features based on prediction results gained with the training dataset. We used prediction accuracy as the performance measure to be maximized during the second stage feature selection.
The list of all the laboratory/imaging tests and other clinical intervention predictors is presented in detail in Table 13 in the Appendix.
3 Data analysis
Table 5 summarizes univariate statistics for demographics, emergency severity score, and chief complaints (ten representative complaints). Table 6 reports bivariate statistics (admission vs. discharge) for categorical variables including chief complaint, emergency severity score, and clinical intervention items. We report odds ratios based on whether a clinical test/intervention is given or a chief complaint is recorded for a patient or not. Table 7 presents bivariate statistics for numerical values from laboratory test results and vital signs. We recognize that in very large samples, p-values from t-tests can misinform regarding the practical significance of a variable [48]. Therefore, we computed a well-established alternative, Hedge’s g, to measure the effect size recorded for the continuous variables. The rule-of-thumb thresholds for interpreting the effect size through the Hedge’s g statistic are as follows: 0.20, small; 0.50, medium; 0.80, large [49]. According to the thresholds, respirations, pulse oximetry, BNP (brain natriuretic peptide), PT/INR/PTT (prothrombin time/international normalized ratio/partial thromboplastin time), lactate blood, and CBC (complete blood count) with differential variables present the small to medium level of effect between the two classes. Due to space constraints and the size of the feature set, we do not present data analysis results for all the variables for all the classification schemes (i.e., C1, C2, and C3). Rather, we selectively present the multivariate analysis statistics for some key features for the 5-class scheme (the C3 classification scheme). The results are reported in detail in the Results section.
4 Results
4.1 Preliminary data analysis results
We provide the univariate and bivariate analysis results obtained from the whole dataset in Table 5 through Table 7.
4.2 C1 classification scheme
As shown in Table 8, right upon arrival to the ED (at door, T1-Arrival), without any clinical information, we can predict admission decision of ED patients with 77.9% (95% confidence interval, CI, of 77.7–78.2) accuracy. While incorporating more information allows the model to enhance its performance (from T1-Arrival through T4-Initial Lab Results), the biggest improvement is made at triage (T2-Triage) where 56.3% of admitted patients are correctly predicted with less than 7% of false positives. There are additional information gains with the first set of laboratory/imaging test orders and other clinical intervention items (T3-Initial Assessment) and laboratory test results (T4-Initial Lab Results) that lead to further improvements in prediction quality. Unlike the admission prediction study conducted at the ED in an Israeli hospital [26], the results of laboratory tests (at T4-Initial Lab Results) seem more informative than the decisions to order specific tests and provide other clinical interventions (at T3-Initial Assessment) to enhance the predictions in our study. For reference, we also compare our prediction performance for the binary classification at triage (C1 at T2-Triage) with other admission prediction models made around triage as well as triage nurse predictions (Table 14) [31, 37, 50, 51].
4.3 C2 classification scheme
Similar to the C1 classification scheme, the C2 class scheme gains the steepest increase in prediction ability at triage (Table 9). It is noticeable that the OU class, being an intermediate class between the IU class and the discharge class, does not seem to have clear clinical distinction compared to the other classes. The laboratory test result items (T4-Initial Lab Results) prove their predictive power at the C2 level. In particular, the precision of classification for the OU class exceeds 50% utilizing laboratory test results, doubling the sensitivity at T2-Triage.
4.4 C3 classification scheme
Predictions on the C3 classification scheme provide the most actionable information among the three classification schemes for the proactive coordination of admission processes. We present prediction and analysis results for the C3 classification scheme from multiple angles to open a rich discussion.
4.4.1 Prediction quality evolvement trajectories
The C3 class classification scheme would provide the most informative results for proactive resource coordination. We choose to report prediction results graphically to clearly depict prediction quality progression for each class (Fig. 4) as well as with a table that reports numerical values of classification sensitivity and precision levels at C3 (Table 15 in the Appendix). Figure 4 reports the sensitivity (Fig. 4a) and precision (Fig. 4b) results for each class. The C3 classification scheme is an imbalanced multiclass classification problem with a small fraction of the patients belonging to minor classes (especially TU and ICU with ~2% and ~4% in the testing sample, respectively), compared to the major class (i.e., discharge with 75.7%). At T1-Arrival, the prediction model fails to detect the minor classes, suggesting that there is insufficient information to identify patients belonging to the minor classes. At T2-Triage, prediction quality varies depending on the class. The highest levels of sensitivity and prediction are obtained for the ICU class. It indicates that ICU patients possess the most distinct features at the triage stage, and even though the number of ICU patients is small (only 3.8%), around 40% of ICU patients can be detected with about 50% precision. Triage information also carries considerable importance for GPU class prediction.
Characterization of progressive nature of disposition decision predictions. Note: Each line represents each class in the C3 classification scheme except the discharge class. The two figures compare prediction model performances (sensitivity and precision, respectively) at the different caregiving epochs
We also observe that predictions of the TU and OU classes are markedly less accurate compared to the ICU and GPU classes at T2-Triage, implying that unlike ICU and GPU patients, TU and OU patients do not have clear clinical trajectory at triage and remain as in-between states, i.e., the TU between the ICU and GPU, and the OU between the GPU and discharge. However, we can recognize that the physicians’ decisions to order certain laboratory/imaging tests and provide other necessary clinical interventions prove useful for predicting the TU class (T3-Initial Assessment). While clinical care intensity and needs of TU patients are difficult to estimate at triage, physicians would start to assess the clinical severity and require care services (especially constant cardiac monitoring) for patients based on triage information and try to differentiate TU patients by ordering laboratory/imaging tests and providing other clinical interventions.
The results in Fig. 4 show that information gained from laboratory test results is generally larger than that from laboratory test order items. The precision levels of prediction are greater than 45.0% in all 5 classes at T4-Initial Lab Results, while sensitivity levels vary. The ICU and GPU classes mark the highest sensitivity levels with more than 42.0% (apart from discharged patients with 96.4%), while 36.4% of TU classes are detected. With the comparatively high-performance levels for the ICU class at T2-Triage, the additional gains of prediction ability for the ICU class at T3-Initial Assessment and T4-Initial Lab Results are not drastic compared to other minor units. The distinct progression behaviours of different classes at C3 clearly indicate that any proactive coordination strategy that utilizes ED disposition decision prediction should consider the different levels of prediction quality obtained at different care epochs for each IU.
Setting each of the four admission classes as a positive class at the C3 classification scheme, the false positive rates at T4-Initial Lab Results are only 1.4%, 1.0%, 4.3%, and 1.4% for the ICU, TU, GPU, and OU classes, respectively. Also, the area under curve (AUC) values are 0.97, 0.95, 0.89, and 0.84 for the four classes, respectively. The AUC value for the discharge class is 0.92. We provide the receiver operating characteristic curves for the five classes at the C3 classification scheme at T4-Initial Lab Results (Fig. 5).
Paired t-tests were run for the five paired observations (five AUC values for the five classes) through the four caregiving epochs to report formal statistical test results for incremental improvement in disposition decision prediction. Fig. 6 provides the forest plots reporting the weights (i.e., the size of the black squares calculated as the inverse of the standard error values) and 95% CI of the mean difference between paired observations (the AUC values for the five classes). The p-values for T1-Arrival to T2-Triage, T2-Triage to T3-Initial Assessment, and T3-Initial Assessment to T4-Initial Lab Results are 0.002, 0.02, and 0.007, respectively. The set of test results conforms to our observations from Fig. 4 and Table 15.
4.4.2 Prediction threshold analysis at C3
It is important to explore how the prediction results can be further exploited to enable the effective operationalization of prediction information. Especially, being a probabilistic classifier, multinomial logistic regression outputs an estimated probability value for each of the classes for a patient as well as a predicted class. The estimated probability values are metrics to measure the level of confidence of membership at each disposition class. By imposing a probability confidence threshold in making a prediction, the model does not make any prediction unless one of the classes has a higher probability value than the threshold. Therefore, as we set higher and higher thresholds, predictions would become more and more reliable with increasing precision levels. Figure 7a, b show behaviours of the sensitivity and precision of prediction respectively for each class among the patients who have higher probability values than the different threshold levels. Figure 7c displays the fraction of patients remaining in the analysis with the different threshold values. For instance, if we impose 60% threshold to the ICU class, the sensitivity of ICU class prediction among the fraction of the patients increases from 45.8% to 62.1%, and the precision would increase from 55.4% to 68.8% while 82.6% of the patients remain (are included) in the analysis. It is noticeable that although the higher threshold values tend to bring higher sensitivity values for the ICU and TU class patients, it does not affect the GPU and OU class patients in the same way. This implies that the predictions of the ICU and TU classes have higher confidence (with higher probability values) than those of the GPU and OU classes because of their clinical distinctiveness.
4.4.3 Feature analysis for C3 classification scheme at T4-Initial Lab Results
We report the most statistically significant features (top 30 variables) at T4-Initial Lab Results for the C3 scheme, based on the chi-square test (Table 10). The study hospital ED has four compartmental care areas. Triage nurses determine the most appropriate care area (e.g., the main ED and fast track) for an ED patient based on the triage information of the patient. This feature presents high importance for disposition decision prediction at C3. It is also notable that 14 laboratory test result features are included in the top 30 list at T4-Initial Lab Results (the “LAB” items in Table 10). The laboratory test results provide detailed information on patients’ conditions that can help predictions at the most granular level (C3). In Table 16, we compare the distributions of informative feature values across the five classes in the C3 classification scheme. The laboratory test result items as well as the primary care area and emergency severity score variables are selected to be presented in the table. A probability value is highlighted in bold when the probability at a minor class exceeds 30%, or the probability of the discharge class surpasses 95%. For instance, it is noticeable that patients with “high panic” results in the troponin I test are likely to be admitted to the TU with 51% probability.
5 Discussion
We attempted to frame ED disposition prediction as an analytics problem, seeking proactive resource allocation and task initiation for potential ED admissions to reduce patient boarding. To the best of our knowledge, this work is the first attempt to define the ED disposition decision prediction as a hierarchical multiclass classification problem, categorizing the admission patients into more detailed classes so that the outcomes of the prediction can become useful for unit-specific proactive coordination across the ED-to-IU workflow. This study shows that in the study hospital, as patient information is accumulated throughout the ED caregiving process, the prediction power gradually increases with unique patterns depending on the target class. It implies that a proper proactive resource allocation and task initiation strategy should vary across the different classes (i.e., destination units) depending on their own uncertainty reduction behaviors.
To discuss the use of prediction information in more detail, we refer back to Table 1, where the general application ideas are introduced along with promising use cases. In our case study hospital, it is encouraging to see that reasonable predictive capability for the four admission classes is obtained with enough lead time for proactive coordination (i.e., around two and a half hours prior to the actual disposition decisions for the admitted patients). This finding shows that there is good potential for realizing the proactive coordination across the ED-to-IU workflow as well as the improved care delivery for patients within an ED, which are briefly listed in Table 1. One application that operationalizes the prediction information is to send an advance bed request signal for a patient to the predicted destination unit when a prediction probability value exceeds a pre-set threshold probability. In this way, a proper inpatient bed can be identified and prepared in advance while the patient is still going through his/her remaining ED caregiving processes. This approach can significantly reduce delay in bed allocation for an admitted patient. In addition, considering the distinct trajectories of prediction capability for patients destined to different IUs, advance bed request signals would be sent to, for example, ICU more often than to TU at triage. A bed manager would wait until more information is revealed and collected (beyond triage) for patients that are likely to be admitted to TU to avoid prediction errors. A data processing/analytics platform is currently under development to be implemented at the study hospital for early inpatient bed preparation and allocation. A similar approach can be applied for providing a better coordinated transport service to admitted patients. In large hospitals, inefficient dispatch of transporters could incur serious delays, and the routing of transporters can be very complex. Therefore, it is important to assign transporters, considering currently waiting admitted patients as well as patients to be admitted soon. By acquiring advance inpatient bed request signals, a transporter dispatcher can have a better sense for performing planned transporter dispatches to enhance operational efficiency. Lastly, the acquisition of advance information on disposition decisions can improve resource allocation in other indirect ways. For instance, hospitals can better balance workload over multiple IUs by proactively mobilizing care providers, responding to projected demand. The advance information can also allow time to prepare spaces in areas that are easily congested in hospitals (such as discharge holding areas).
This study also provides insights into the relationship between physicians’ disposition decisions and clinical laboratory test result values through large scale data analysis.
6 Limitations
One of the limitations of this work is that we cannot guarantee whether the models have exploited the collected information to the greatest extent. For instance, while we categorized the numerical variables such as vital signs and laboratory test results into finite levels, more advanced data driven methodologies, such as deep learning approaches, would be able to better extract features by thoroughly exploiting interrelationships between the numerical variables. These approaches might compromise the repeatability and consistency of prediction, but the methods are rapidly becoming mature with technical advances. Another limitation of our work is that there is other information in the patient’s electronic health record that could be included in the models to further improve the predictive power of the models. In particular, physician “notes” record clinically significant information in various formats including text. Since the notes contain refined information that comes from the interpretation of the clinical examination of patients, the inclusion of features derived from physician notes can probably improve the prediction quality for disposition decisions. In addition, considering that radiology and other imaging tests are one of the most heavily utilized diagnostic items in the ED, future works should incorporate imaging data and/or results summaries from clinicians, when available in a timely fashion, into disposition prediction models to improve accuracy. Besides the clinical information, socio-demographic information (such as place of residence and income) can also contribute to improving disposition decision prediction quality. While this work has primarily focused on exploiting clinical information readily available in most EHR systems for building prediction models, the impact of the inclusion of socio-demographic information is worth investigating in future works. Finally, even though the study is conducted at a large level-1 trauma center using a relatively large dataset from a two-year period, it would be important to replicate the study at other hospitals. For example, while we could thoroughly incorporate all the data items into the models due to the well-developed information system adopted by the study hospital, this may not be feasible in other hospitals. However, the accrual of clinical information to power the proposed patient disposition prediction models would be comparable across most EDs. We captured the general caregiving steps in the ED for analytical discussion on the predictive capability of ED patient information in predicting disposition decisions.
7 Conclusion
This work builds on a growing body of academic and industrial literature on the usefulness and feasibility of proactive coordination in healthcare that can be empowered by predictive analytics. A proper multiclass prediction framework that considers the actual patient flow, resource requirements, and resource management practices around the ED-to-IU workflow can help with proactive early task initiation and resource allocation for admitted ED patients to reduce patient boarding. Since reactive processes are prevalent in current ED-to-IU workflow operations across most hospitals (including hospital admission approval, administrative procedures, bed management, transporter assignment and so on) the prediction results could contribute in different forms and ways depending on the specific ED-to-IU operations and practices at different hospitals.
Notes
In Figure 1b, we conservatively exclude the case of having only one unoccupied bed to account for any possibility that a bed is temporarily unavailable due to, for example, infection concerns from a fellow roomed patient and so on.
References
Liu S, Hobgood C, Brice JH (2003) Impact of critical bed status on emergency department patient flow and overcrowding. Acad Emerg Med 10:382–385
Hoot NR, Aronsky D (2008) Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med 52:126–136
Pines JM, Batt RJ, Hilton JA, Terwiesch C (2011) The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med 58:331–340
Carter EJ, Pouch SM, Larson EL (2014) The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh 46:106–115
King DL, Ben-Tovim DI, Bassham J (2006) Redesigning emergency department patient flows: application of lean thinking to health care. Emerg Med Australas 18:391–397
Kelly AM, Bryant M, Cox L, Jolley D (2007) Improving emergency department efficiency by patient streaming to outcomes-based teams. Aust Health Rev 31:16–21
Ben-Tovim DI, Bassham JE, Bennett DM, Dougherty ML, Martin MA, O’Neill SJ, Sincock JL, Szwarcbord MG (2008) Redesigning care at the Flinders medical Centre: clinical process redesign using “lean thinking”. Med J Aust 188:S27cS31
Rodi SW, Grau MV, Orsini CM (2006) Evaluation of a fast track unit: alignment of resources and demand results in improved satisfaction and decreased length of stay for emergency department patients. Qual Manag Health Care 15:163–170
Considine J, Kropman M, Kelly E, Winter C (2008) Effect of emergency department fast track on emergency department length of stay: a case-control study. Emerg Med J 25:815–819
Dickson EW, Singh S, Cheung DS, Wyatt CC, Nugent AS (2009) Application of lean manufacturing techniques in the emergency department. J Emerg Med 37:177–182
Holden RJ (2011) Lean thinking in emergency departments: a critical review. Ann Emerg Med 57:265–278
Fatovich DM, Nagree Y, Sprivulis P (2005) Access block causes emergency department overcrowding and ambulance diversion in Perth, Western Australia. Emerg Med J 22:351–354
Pines JM, Hilton JA, Weber EJ, Alkemade AJ, Al Shabanah H, Anderson PD, Bernhard M, Bertini A, Gries A, Ferrandiz S, Kumar VA (2011) International perspectives on emergency department crowding. Acad Emerg Med 18:1358–1370
Pines JM, Bernstein SL (2015) Solving the worldwide emergency department crowding problem–what can we learn from an Israeli ED? Isr J Health Policy 4:52
Van Der Linden MC, Khursheed M, Hooda K, Pines JM, Van Der Linden N (2007) Two emergency departments, 6000 km apart: differences in patient flow and staff perceptions about crowding. Int Emerg Nurs 35:30–36
Rabin E, Kocher K, McClelland M, Pines J, Hwang U, Rathlev N, Asplin B, Trueger NS, Weber E (2012) Solutions to emergency department ‘boarding’ and crowding are underused and may need to be legislated. Health Affair 31:1757–1766
Kim MJ, Park JM, Je SM, You JS, Park YS, Chung HS, Chung SP, Lee HS (2012) Effects of a short text message reminder system on emergency department length of stay. Int J Med Inform 81:296–302
Zhou JC, Pan KH, Zhou DY, Zheng SW, Zhu JQ, Xu QP, Wang CL (2012) High hospital occupancy is associated with increased risk for patients boarding in the emergency department. Am J Med 125:416–4e1
Centers for Medicare and Medicaid Services (2018) Hospital Compare datasets. https://data.medicare.gov/data/hospital-compare/. Accessed December 11, 2018
Saghafian S, Hopp WJ, Van Oyen MP, Desmond JS, Kronick SL (2012) Patient streaming as a mechanism for improving responsiveness in emergency departments. Oper Res 60:1080–1097
Shi P, Chou MC, Dai JG, Ding D, Sim J (2015) Models and insights for hospital inpatient operations: time-dependent ED boarding time. Manag Sci 62:1–28
Osorio C, Bierlaire M (2009) An analytic finite capacity queueing network model capturing the propagation of congestion and blocking. Eur J Oper Res 196:996–1007
Peck JS, Benneyan JC, Nightingale DJ, Gaehde SA (2012) Predicting emergency department inpatient admissions to improve same-day patient flow. Acad Emerg Med 19:E1045–E1054
Peck JS, Benneyan JC, Nightingale DJ, Gaehde SA (2014) Characterizing the value of predictive analytics in facilitating hospital patient flow. IIE Trans Healthc Syst Eng 4:135–143
Qiu S, Chinnam RB, Murat A, Batarse B, Neemuchwala H, Jordan W (2015) A cost sensitive inpatient bed reservation approach to reduce emergency department boarding times. Health Care Manag Sci 18:67–85
Barak-Corren Y, Israelit SH, Reis BY (2017) Progressive prediction of hospitalisation in the emergency department: uncovering hidden patterns to improve patient flow. Emerg Med J 34:308–314
Bayley MD, Schwartz JS, Shofer FS, Weiner M, Sites FD, Traber KB, Hollander JE (2005) The financial burden of emergency department congestion and hospital crowding for chest pain patients awaiting admission. Ann Emerg Med 45:110–117
Yancer DA, Foshee D, Cole H, Beauchamp R, de la Pena W, Keefe T, Smith W, Zimmerman K, Lavine M, Toops B (2006) Managing capacity to reduce emergency department overcrowding and ambulance diversions. Joint Comm J Qual Patient Saf 32:239–245
Thomas BG, Bollapragada S, Akbay K, Toledano D, Katlic P, Dulgeroglu O, Yang D (2013) Automated bed assignments in a complex and dynamic hospital environment. Interfaces 43:435–448
Tortorella F, Ukanowicz D, Douglas-Ntagha P, Ray R, Triller M (2013) Improving bed turnover time with a bed management system. J Nurs Admin 43:37–43
Sun Y, Heng BH, Tay SY, Seow E (2011) Predicting hospital admissions at emergency department triage using routine administrative data. Acad Emerg Med 18:844–850
Boyle J, Jessup M, Crilly J, Green D, Lind J, Wallis M, Miller P, Fitzgerald G (2012) Predicting emergency department admissions. Emerg Med J 29:358–365
Cameron A, Rodgers K, Ireland A, Jamdar R, McKay GA (2015) A simple tool to predict admission at the time of triage. Emerg Med J 32:174–179
Dinh MM, Russell SB, Bein KJ, Rogers K, Muscatello D, Paoloni R, Hayman J, Chalkley DR, Ivers R (2016) The Sydney triage to admission risk tool (START) to predict emergency department disposition: a derivation and internal validation study using retrospective state-wide data from New South Wales, Australia. BMC Emerg Med 16:46
Handly N, Thompson DA, Venkat A (2013) Derivation and validation of a hospital admission prediction model adding coded chief complaint to demographic, emergency department operational and patient acuity data available at emergency department triage using neural net methodology. Ann Emerg Med 62:S138
Kim SW, Li JY, Hakendorf P, Teubner DJ, Ben-Tovim DI, Thompson CH (2014) Predicting admission of patients by their presentation to the emergency department. Emerg Med Australas 26:361–367
Walsh P, Cunningham P, Rothenberg SJ, O'Doherty S, Hoey H, Healy R (2004) An artificial neural network ensemble to predict disposition and length of stay in children presenting with bronchiolitis. Eur J Emerg Med 11:259–264
Gordon PC, Wanklin JM, Harvey NH, Hatcher GH (1966) An approach to patient care classification. Can Med Assoc J 95:1228–1236
Bhutta ZA, Khan I, Salat S, Raza F, Ara H (2004) Reducing length of stay in hospital for very low birthweight infants by involving mothers in a stepdown unit: an experience from Karachi (Pakistan). BMJ 329:1151–1155
Marra AR, D'Arco C, de Arruda Bravim B, Martino MD, Correa L, Silva CV, Lamblet LC, Junior MS, de Lima G, Guastelli LR, Barbosa L (2008) Controlled trial measuring the effect of a feedback intervention on hand hygiene compliance in a step-down unit. Infect Cont Hosp Epidemiol 29:730–735
van Sluisveld N, Hesselink G, van der Hoeven JG, Westert G, Wollersheim H, Zegers M (2015) Improving clinical handover between intensive care unit and general ward professionals at intensive care unit discharge. Intensive Care Med 41:589–604
Silla CN Jr, Freitas AA (2011) A survey of hierarchical classification across different application domains. Data Min Knowl Disc 22:31–72
Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE (2011) Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. Springer Science & Business Media
Centers for Medicare and Medicaid Services (2017) National Health Expenditure Data. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/index.html. Accessed December 1, 2018
Gilboy N, Tanabe T, Travers D, Rosenau AM (2011) Emergency severity index (ESI): a triage tool for emergency department care, version 4. Agency for Healthcare Research and Quality Publication No, Rockville, pp 12–0014
National Library of Medicine (2015) Vital signs. https://medlineplus.gov/vitalsigns.html. Accessed December 15, 2018
Forman G (2003) An extensive empirical study of feature selection metrics for text classification. J Mach Learn Res 3:1289–1305
Lin M, Lucas HC Jr, Shmueli G (2013) Research commentary—too big to fail: large samples and the p-value problem. Inf Syst Res 24(4):906–917
Cohen J (2013) Statistical power analysis for the behavioral sciences. Routledge
Stover-Baker B, Stahlman B, Pollack M (2012) Triage nurse prediction of hospital admission. J Emerg Nurs 38:306–310
Arslanian-Engoren C (2004) Do emergency nurses' triage decisions predict differences in admission or discharge diagnoses for acute coronary syndromes? J Cardiovasc Nurs 19:280–286
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Lee, SY., Chinnam, R.B., Dalkiran, E. et al. Prediction of emergency department patient disposition decision for proactive resource allocation for admission. Health Care Manag Sci 23, 339–359 (2020). https://doi.org/10.1007/s10729-019-09496-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10729-019-09496-y