Abstract
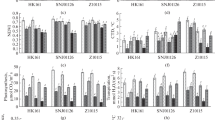
The objective of this study was to evaluate the effects of abscisic acid (ABA) related to the increase of water-stress tolerance in two drought contrasting maize hybrids: DKB 390 (tolerant) and BRS 1030 (sensitive). The characterization of water status (pre-dawn leaf water potential, Ψpd; midday leaf water potential, Ψmd and stem water potential, Ψst) and antioxidant enzyme activity was conducted on greenhouse grown plants. The ABA, hydrogen peroxide (H2O2), and malondialdehyde (MDA) contents were also analyzed. Water deficit was imposed for 10 days at the flowering stage and a dosage of 100 μM ABA was applied to plant canopy. Measurements were taken during 10 days after the water recovery. With 5 days of stress, the tolerant hybrid showed lower MDA content, decrease in the water status, and higher activity of the enzymes superoxide dismutase, catalase, ascorbate peroxidase, as well as guaiacol, glutathione reductase, dehydroascorbate reductase, polyphenol oxidase, and l-phenylalanine ammonia-lyase, as compared to the sensitive hybrid. With 10 days of stress, DKB 390 had a decrease in the activity of enzymes whereas BRS 1030 showed a higher activity. In addition, the latter showed greater amounts of H2O2 and MDA. ABA application led to a higher tolerance only in DKB 390, due to the increase of water status and the enzymatic activity, mainly the catalase.




Similar content being viewed by others
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ali Q, Ashraf M (2011) Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J Agron Crop Sci 197:258–271
Anjum SA, Farooq M, Wang LC, Xue LL, Wang SG, Wang S, Zhang S, Chen M (2011a) Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycinebetaine under drought conditions. Plant Soil Environ 57:326–331
Anjum SA, Wang L, Farooq M, Xue L, Ali S (2011b) Fulvic acid application improves the maize performance under well-watered and drought conditions. J Agron Crop Sci 197:409–417
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM (2011c) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197:177–185
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Biol 55:373–399
Araus JL, Sànchez C, Edmeades GO (2011) Phenotyping maize for adaptation to drought. In: Monneveux P, Ribaut JM (eds) Drought phenotyping in crops: from theory to practice. CGIAR Generation Challenge Programme, Texcoco, pp 263–283
Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Díaz M, Tognoni F, Pardossi A (2003) Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Sci 165:671–679
Bano A, Yasmeen S (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42:2579–2587
Bano A, Ullah F, Nosheen A (2012) Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant Soil Environ 58:181–185
Begg JE, Turner NC (1970) Water potential gradients in field tobacco. Plant Physiol 46:343–346
Bettaieb I, Hamrouni-Sellami I, Bourgou S, Limam F, Marzouk B (2011) Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia offcinalis L. Acta Physiol Plant 33:1103–1111
Bradford M (1976) Rapid and quantitative method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–252
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzimol 52:302–310
Cahill DM, McComb JA (1992) A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinnamomi. Physiol Mol Plant Pathol 40:315–332
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide—scavenging enzymes in germination wheat seeds. J Exp Bot 44:127–132
Choné X, Leeuwen CV, Dubourdieu D, Gaudillere JP (2001) Stem water potential is a sensitive indicator of grapevine water status. Ann Bot 87:477–483
Christmann A, Hoffmann T, Teplova I, Grill E, Muller A (2005) Generation of actives pools of abscísico acid revealed by in vivo imaging of water-stressed Arabidopsis. Plant Physiol 137:209–219
Correâ de Souza T, Mauro de Castro E, César Magalhães P, De Oliveira Lino L, Trindade Alves E, Emílio Pereira de Albuquerque P (2013a) Morphophysiology, morphoanatomy, and grain yield under field conditions for two maize hybrids with contrasting response to drought stress. Acta Physiol Plant 35:3201–3211
Correâ de Souza T, César Magalhães P, Mauro de Castro E, Emílio Pereira de Albuquerque P, Marabesi MA (2013b) The influence of ABA on water relation, photosynthesis parameters, and chlorophyll fluorescence under drought conditions in two maize hybrids with contrasting drought resistance. Acta Physiol Plant 35:515–527
Chugh V, Kaur N, Gupta AK (2011) Evaluation of oxidative stress tolerance in maize (Zea mays L.) seedlings in response to drought. Indian J Biochem Biophys 48:47–53
DaCosta M, Huang B (2007) Drought survival and recuperative ability of bentgrass species associated with changes in abscísico acid and cytokinin production. Am Soc Hort Sci 132:133–141
Darkó É, Fodor J, Dulai S, Ambrus H, Szenzenstein A, Király Z, Barnabás B (2011) Improved cold and drought tolerance of doubled haploid maize plants selected for resistance to prooxidant tert-butyl hydroperoxide. J Agron Crop Sci 197:454–465
Dolatabadian A, Modarres-Sanavy SAM, Sharifi M (2009) Alleviation of water deficit stress effects by foliar application of ascorbic acid on Zea mays L. J Agron Crop Sci 195:347–355
Fan XW, Li FM, Song L, Xiong YC, An LZ, Jia Y, Fang XW (2009) Defense strategy of old and modern spring wheat varieties during soil drying. Physiol Plant 136:310–323
Farooq M, Wahid A, Kokayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Farré I, Faci J-M (2009) Deficit irrigation in maize for reducing agricultural water use in a mediterranean environment. Agric Water Manag 96:383–394
Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant 51:98–103
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I: occurrence in higher plants. Plant Physiol 59:309–314
Gholizadeh A (2010) Anti-oxidation profile in the leaves of maize inbreds: elevation in the activity of phenylalanine ammonia lyase under drought stress. J Plant Sci 5:137–145
Guóth A, Tari I, Gallé A, Csiszár J, Pécsváradi A, Cseuz L, Erdei L (2009) Comparison of the drought stress responses of tolerant and sensitive wheat cultivars during grain filling: changes in flag leaf photosynthetic activity, ABA levels, and grain yield. J Plant Growth Regul 28:167–176
Havir EA, Mchale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 25:85–92
Hu XL, Jiang M, Zhang A, Lu J (2005) Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 223:57–68
Hura T, Hura K, Grzesiak S (2008) Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J Agron Crop Sci 194:104–112
Jiang M, Zhang J (2002) Involvement of plasma membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215:1022–1030
Jiang M, Zhang J (2003) Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defense in leaves of maize seedlings. Plant Cell Environ 26:929–939
Kar ME, Mishra D (1976) Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Karuppanapandian T, Moon J-C, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5:709–725
Kellos T, Tímár I, Silágyi V, Szalai G, Galiba G, Kocsy G (2008) Stress hormones and abiotic stress have different effects on antioxidants in maize lines with different sensitivity. Plant Biol 10:563–572
Kudoyarova G, Veselova S, Hartung W, Farhutdinov R, Veselov D, Sharipova G (2011) Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 233:87–94
Lin J, Wang G (2002) Doubled CO2 could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat. Plant Sci 163:627–637
Liu F, Jensen CR, Andersen MN (2005) A review of drought adaptation in crop plants: changes in vegetative and reproductive physiology induced by ABA-based chemicals signals. Aust J Agric Res 56:1245–1252
Lu S, Su W, Li H, Guo Z (2009) Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2- and NO-induced antioxidant enzyme activies. Plant Physiol Biochem 47:132–138
Martins AO (2012) Genetic and physiological inferences of drought tolerance in maize.Thesis, Universidade Estadual Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes-RJ, Brazil
Medici LO, Machado AT, Azevedo RA, Pimentel C (2003/2004) Glutamine synthetase activity relative water content and water potential in maize submitted to drought. Biol Plant 47:301–304
Mitler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Moussa HR, Abdel-Aziz SM (2008) Comparative response of drought tolerant and sensitive maize genotypes to water stress. Aust J Crop Sci 1:31–36
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbato-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pourcel L, Routaboul J-M, Cheynier V, Lepiniec L, Debeaujon I (2006) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36
Queval G, Hager J, Gakière B, Noctor G (2008) Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J Exp Bot 59:135–146
Sánchez-Rodríguez E, Moreno DA, Ferreres F, Rubio-Wilhelmi M del-M (2011) Differential responses of five cherry tomato varieties to water stress: changes on phenolic metabolites and related enzymes. Phytochemistry 72:723–729
Szalai G, Hellos T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under stress conditions. J Growth Regul 28:66–80
Travaglia C, Reinoso H, Bottini R (2009) Application of abscisic acid promotes yield in field-cultured soybean by enhancing production of carbohydrates and their allocation in seed. Crop Pasture Sci (Aust J Agric Res) 60:1131–1136
Travaglia C, Reinoso H, Cohen A, Luna C, Tommasino E, Castillo C, Bottini R (2010) Exogenous ABA increases yield in field-grown wheat with a moderate water restriction. J Plant Growth Regul 29:366–374
Travaglia C, Balboa G, Espósito G, Reinoso H (2012) ABA action on the production and redistribution of field-grown maize carbohydrates in semiarid regions. J Growth Regul 67:27–34
Yang J, Zhang J, Ye Y, Wang Z, Zhu Q, Liu L (2004) Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant Cell Environ 27:1055–1064
Ye N, Zhu G, Liu Y, Li Y, Zhang J (2011) ABA Controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol 52:689–698
Acknowledgments
The authors would like to thank Capes for the scholarship; Centro Nacional de Pesquisa de Milho e Sorgo, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Laboratório de Anatomia Vegetal da Universidade Federal de Lavras for providing the facilities and materials that made this research possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, T.C., Magalhães, P.C., de Castro, E.M. et al. ABA application to maize hybrids contrasting for drought tolerance: changes in water parameters and in antioxidant enzyme activity. Plant Growth Regul 73, 205–217 (2014). https://doi.org/10.1007/s10725-013-9881-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9881-9