Abstract
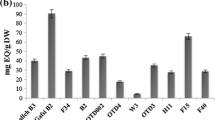
Secondary metabolites (SMs), such as alkaloids and raffinose oligosaccharides (RFOs), play important roles in plant physiology. Although alkaloid and RFO phenotypic variation has been reported for yellow lupin (Lupinus luteus L.), most evaluations have used a reduced number of accessions; thus, limiting the understanding of accumulation patterns and variation ranges. The main goal of this research was to assess alkaloid and RFO content in a diverse L. luteus sample to understand possible SM accumulation patterns across this legume species. Eighteen yellow lupin accessions were analyzed using high performance thin layer chromatography to provide insights on seed and leaf RFO and alkaloid phenotypic variation. Co-dominant markers (170) were used to examine genetic relationships among L. luteus accessions and possible accumulation patterns across closely related genotypes. Significant differences were observed for seed and leaf RFOs. Total seed RFO accumulation ranged from 79.738 to 131.079 mg g−1. Raffinose, stachyose, and verbascose were observed in all genotypes’ seeds, but at different RFO concentrations. Raffinose was the only RFO detected in leaves (2.793–0.4224 mg g−1). Total alkaloid accumulation ranged from 0.22 to 15.12 and 0.00 to 8.007 mg g−1 for seeds and leaves, respectively. Lupinine, sparteine, and gramine were observed in seeds and leaves, and showed a wide range of variation. Neighbor-Joining (NJ) analysis showed an apparent pattern of seed alkaloid accumulation, most likely due to domestication events. However, high RFO accumulating accessions were scattered across the NJ tree. Alkaloid and RFO significant phenotypic variation will not only help to understand the roles of these SMs in L. luteus, but also to uncover the genetic basis behind their accumulation.


Similar content being viewed by others
References
Adhikari K, Edwards O, Wang S, Ridsdill-Smith T, Buirchell B (2012) The role of alkaloids in conferring aphid resistance in yellow lupin (Lupinus luteus L.). Crop Pasture Sci 63:444–451
Agatonovic-Kustrin S, Loescher C, Morton D (2015) Quantitative errors and uncertainty in high-performance thin-layer chromatographic method for the quality assessment of Calendula offcinalis plant extracts. JPC J Planar Chromat 28:213–217
Allen JG (1998) Toxins and lupinosis. In: Glastones JS, Atkins CA, Hamblin C (eds) Lupines as a crop plants: biology, production and utilization. CAB International, University Press, Cambridge, pp 411–428
Andersen KE, Bjergegaard C, Møller P, Sørensen JC, Sørensen H (2005) Compositional variations for α-galactosides in different species of Leguminosae, Brassicaceae, and barley: a chemotaxonomic study based on chemometrics and high-performance capillary electrophoresis. J Agr Food Chem 53:5809–5817
Bachmann M, Keller F (1995) Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. (Inter- and Intracellular Compartmentation). Plant Physiol 109:991–998
Banuelos Pineda J, Nolasco Rodriguez G, Monteon JA, Garcia Lopez PM, Ruiz Lopez MA, Garcia Estrada J (2005) Histological evaluation of brain damage caused by crude quinolizidine alkaloid extracts from lupines. Histol Histopathol 20:1147–1153
Berger JD, Ludwig C (2014) Contrasting adaptive strategies to terminal drought-stress gradients in Mediterranean legumes: phenology, productivity, and water relations in wild and domesticated Lupinus luteus L. J Exp Bot 65:6219–6229
Berger JD, Buirchell BJ, Luckett DJ, Nelson MN (2012) Domestication bottlenecks limit genetic diversity and constrain adaptation in narrow-leafed lupin (Lupinus angustifolius L.). Theor Appl Genet 124:637–652
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95:4126–4133
Brotman Y, Riewe D, Lisec J, Meyer RC, Willmitzer L, Altmann T (2011) Identification of enzymatic and regulatory genes of plant metabolism through QTL analysis in Arabidopsis. J Plant Physiol 168:1387–1394
Bunsupa S, Katayama K, Ikeura E, Oikawa A, Toyooka K, Saito K, Yamazaki M (2012) Lysine decarboxylase catalyzes the first step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid production in leguminosae. Plant Cell 24:1202–1216
Chen YH, Gols R, Benrey B (2015) Crop domestication and its impact on naturally selected trophic interactions. Annu Rev Entomol 60:35–58
Chilomer K, Zaleska K, Ciesiolka D, Gulewicz P, Frankiewicz A, Gulewicz K (2010) Changes in the alkaloid, alpha-galactoside and protein fractions content during germination of different lupin species. Acta Soc Bot Pol 79:11–20
Chow CY, Kelsey KJP, Wolfner MF, Clark AG (2016) Candidate genetic modifiers of retinitis pigmentosa identified by exploiting natural variation in Drosophila. Hum Mol Genet 25:651–659
Cowling W, Gladstones J (2000) Lupin breeding in Australia. In: Knight R (ed) Linking research and marketing opportunities for pulses in the 21st century. Springer, Berlin, pp 541–547
Doyle JJ (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Frias J, Vidal-Valverde C, Kozlowska H, Gorecki R, Honke J, Hedley C (1996) Evolution of soluble carbohydrates during the development of pea, faba bean and lupin seeds. Z Lebensm Unters Forch 203:27–32
Ganzera M, Kruger A, Wink M (2010) Determination of quinolizidine alkaloids in different Lupinus species by NACE using UV and MS detection. J Pharmaceut Biomed 53:1231–1235
Gardiner MR (1967) Lupinosis. Adv Vet Sci 11:85–138
Gladstones JS (1974) Lupins of the Mediterranean region and Africa West Aust Dep Agric, Tech Bull No 26
Gladstones JS, Atkins C, Hamblin J (1998) Distribution, origin, taxonomy, history and importance. In: Gladstones JS, Atkins CA, Hamblin J (eds) Lupins as crop plants: biology, production and utilization. CAB international, Wallingford, UK, pp 1–77
Górecki R, Piotrowicz-Cieślak A, Lahuta L, Obendorf R (1997) Soluble carbohydrates in desiccation tolerance of yellow lupin seeds during maturation and germination. Seed Sci Res 7:107–116
Grant T, Dami I, Ji T, Scurlock D, Streeter J (2009) Variation in leaf and bud soluble sugar concentration among Vitis genotypes grown under two temperature regimes. Can J Plant Sci 89:961–968
Grimbs A et al (2017) Bioactivity in Rhododendron: a systemic analysis of antimicrobial and cytotoxic activities and their phylogenetic and phytochemical origins Front. Plant Sci 8:551
Han IH, Baik B-K (2006) Oligosaccharide Content and Composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chem 83:428–433
Hill CB, Taylor JD, Edwards J, Mather D, Langridge P, Bacic A, Roessner U (2015) Detection of QTL for metabolic and agronomic traits in wheat with adjustments for variation at genetic loci that affect plant phenology. Plant Sci 233:143–154
Hondelmann W (1984) The lupin—ancient and modern crop plant. Theor Appl Genet 68:1–9
Kaale E, Risha P, Layloff T (2011) TLC for pharmaceutical analysis in resource limited countries. J Chromatogr A 1218:2732–2736
Kamel KA, Święcicki W, Kaczmarek Z, Barzyk P (2016) Quantitative and qualitative content of alkaloids in seeds of a narrow-leafed lupin collection. Genet Resour Crop Evol 63:711–719. https://doi.org/10.1007/s10722-015-0278-7
Keurentjes JJB et al (2006) The genetics of plant metabolism. Nat Genet 38:842–849
Kordan B, Dancewicz K, Wróblewska A, Gabryś B (2012) Intraspecific variation in alkaloid profile of four lupine species with implications for the pea aphid probing behaviour. Phytochem Lett 5:71–77
Kroc M, Rybiński W, Wilczura P, Kamel K, Kaczmarek Z, Barzyk P, Święcicki W (2017) Quantitative and qualitative analysis of alkaloids composition in the seeds of a white lupin (Lupinus albus L.) collection. Genet Resour Crop Evol 64:1853–1860
Kumar D, Kumar R, Singh B, Ahuja PS (2015) Reproducible reversed-phase high-performance thin-layer chromatography-based quality-control method for the endangered medicinal plant Picrorhiza kurroa Royle ex Benth. JPC J Planar Chromat 28:256–261
Lee MJ, Pate JS, Harris DJ, Atkins CA (2007) Synthesis, transport and accumulation of quinolizidine alkaloids in Lupinus albus L. and L. angustifolius L. J Exp Bot 58:935–946
Lopes MS et al (2015) Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J Exp Bot 66:3477–3486
Magalhães SC, Fernandes F, Cabrita AR, Fonseca AJ, Valentão P, Andrade PB (2017) Alkaloids in the valorization of European Lupinus spp. seeds crop. Ind Crop Prod 95:286–295
Martínez-Villaluenga C, Frías J, Vidal-Valverde C (2006) Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of α-galactosides. Food Chem 98:291–299
Martínez-Villaluenga C, Frías J, Vidal-Valverde C (2008) Alpha-galactosides: antinutritional factors or functional ingredients? Crit Rev Food Sci Nutr 48:301–316
Martínez-Villaluenga C, Frías J, Vidal-Valverde C (2005) Raffinose family oligosaccharides and sucrose contents in 13 Spanish lupin cultivars. Food Chem 91:645–649
Maureira-Butler IJ, Udall JA, Osborn TC (2007) Analyses of a multi-parent population derived from two diverse alfalfa germplasms: testcross evaluations and phenotype-DNA associations. Theor Appl Genet 115:859–867
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450
Moore BD, Andrew RL, Külheim C, Foley WJ (2014) Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol 201:733–750
Mundree SG et al (2002) Physiological and molecular insights into drought tolerance. Afr J Biotechnol 1:28–38
Muzquiz M, Varela A, Burbano C, Cuadrado C, Guillamón E, Pedrosa M (2012) Bioactive compounds in legumes: pronutritive and antinutritive actions. Implic Nutr Health Phytochem Rev 11:227–244
Osorio CE, Udall JA, Salvo-Garrido H, Maureira-Butler IJ (2018) Development and characterization of InDel markers for Lupinus luteus L. (Fabaceae) and cross-species amplification in other Lupin species. Electron J Biotechn 31:44–47
Pandey DK, Dey A (2016) A validated and densitometric HPTLC method for the simultaneous quantification of reserpine and ajmalicine in Rauvolfia serpentina and Rauvolfia tetraphylla. Rev Bras Farmacogn 26:553–557
Panikulangara TJ, Eggers-Schumacher G, Wunderlich M, Stransky H, Schöffl F (2004) Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiol 136:3148–3158
Parra Gonzalez LB, Straub SC, Doyle JJ, Ortega PE, Salvo-Garrido HE, Maureira-Butler IJ (2010) Development of microsatellite markers in Lupinus luteus (Fabaceae) and cross-species amplification in other lupine species. Am J Bot 97:e72–74
Parra-Gonzalez LB, Aravena-Abarzúa GA, Navarro-Navarro CS, Udall JA, Maughan J, Peterson LM, Salvo-Garrido HE, Maureira-Butler IJ (2012) Yellow lupin (Lupinus luteus L.) transcriptome sequencing: molecular marker development and comparative studies. BMC Genom 13:425
Peterbauer T et al (2001) Analysis of the Raffinose family oligosaccharide pathway in pea seeds with contrasting carbohydrate composition. Plant Physiol 127:1764–1772
Peterbauer T, Mach L, Mucha J, Richter A (2002) Functional expression of a cDNA encoding pea (Pisum sativum L.) raffinose synthase, partial purification of the enzyme from maturing seeds, and steady-state kinetic analysis of raffinose synthesis. Planta 215:839–846
Petterson D (1998) Composition and food uses of lupin. In: Gladstones J, Atkins C, Wallingford H (eds) Lupins as crop plants: biology, production, and utilization. CAB Int, UK, pp 353–384
Pichersky E, Lewinsohn E (2011) Convergent evolution in plant specialized metabolism. Annu Rev Plant Biol 62:549–566
Pluskota WE, Szablińska J, Obendorf RL, Górecki RJ, Lahuta LB (2015) Osmotic stress induces genes, enzymes and accumulation of galactinol, raffinose and stachyose in seedlings of pea (Pisum sativum L.). Acta Physiol Plant 37:156
Pothier J, Cheav SL, Galand N, Dormeau C, Viel C (1998) A comparative study of the effects of sparteine, lupanine and lupin extract on the central nervous system of the mouse. J Pharm Pharmacol 50:949–954
Rahman MA, Thomson MJ, Shah-E-Alam M, de Ocampo M, Egdane J, Ismail AM (2016) Exploring novel genetic sources of salinity tolerance in rice through molecular and physiological characterization. Ann Bot 117:1083–1097
Renger B, Végh Z, Ferenczi-Fodor K (2011) Validation of thin layer and high performance thin layer chromatographic methods. J Chromatog A 1218:2712–2721
Ridsdill-Smith T, Edwards O, Wang SF, Ghisalberti E, Reidy-Crofts J (2004) Aphid responses to plant defensive compounds in lupins. In: Simon JC, Dedryver CA, Rispe C, Hullé M (eds) Aphids in a new millennium. Institut National de la Recherche Agronomique, Paris, pp 491–497
Seigler DS (2012) Plant secondary metabolism. Springer, New York, p 759p
Selvaraj MG et al (2017) Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol J 15:1465–1477
Senguttuvan J, Subramaniam P (2016) HPTLC Fingerprints of various secondary metabolites in the traditional medicinal herb Hypochaeris radicata L. J Bot 2016:5429625
Shewiyo DH, Kaale E, Risha PG, Dejaegher B, Smeyers-Verbeke J, Heyden YV (2012) HPTLC methods to assay active ingredients in pharmaceutical formulations: a review of the method development and validation steps. J Pharmaceut Biomed 66:11–23
Swiecicki W, Jach K (1980) Variation and evolution of alkaloid complex in yellow lupine (Lupinus luteus L.) during domestication. Acta Agrobot 33:177–195
Thenmozhi S, Priya ML, Saraswathi G, Dwivedi S, Subasini U (2014) Chromatographic fingerprint analysis of leaf extracts of Vitex leucoxylon Linn. by HPTLC technique. Int J Pharma Teach Pract 5:930–934
Wink M (1988) Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor Appl Genet 75:225–233
Wink M (2003) Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64:3–19
Wink M (2013) Evolution of secondary metabolites in legumes (Fabaceae). S Afr J Bot 89:164–175
Wink M, Mohamed GIA (2003) Evolution of chemical defense traits in the Leguminosae: mapping of distribution patterns of secondary metabolites on a molecular phylogeny inferred from nucleotide sequences of the rbcL gene. Biochem Syst Ecol 31:897–917
Wink M, Witte L (1984) Turnover and transport of quinolizidine alkaloids. Diurnal fluctuations of lupanine in the phloem sap, leaves and fruits of Lupinus albus L. Planta 161:519–524
Wolko B, Clements J, Naganowska B, Nelson M, Ha Yang (2011) Lupinus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Springer, Berlin, pp 153–206
Acknowledgments
This research was founded by the National Commission for Scientific & Technological Research (FONDECYT Project 3140064). The authors thank INIA Carillanca for their contribution in the use of experimental fields and infrastructure.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Osorio, C.E., Amiard, V.S.E., Aravena-Calvo, J. et al. Chromatographic fingerprinting of Lupinus luteus L. (Leguminosae) main secondary metabolites: a case of domestication affecting crop variability. Genet Resour Crop Evol 65, 1281–1291 (2018). https://doi.org/10.1007/s10722-018-0613-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-018-0613-x