Abstract
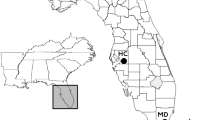
Reintroductions—captive-born animals introduced into the species’ original distribution area—and translocations—free-living animals transferred to another location within the historical distribution area—are important conservation strategies for endangered species. Genetic analyses of 239 individuals from unmanaged, translocated and reintroduced populations of Leontopithecus rosalia were performed using 14 microsatellites. These samples were collected during two periods: (a) 1996–1997 (historic), when individuals were translocated and reintroduced into forest fragments in the lowland Atlantic Forest, and (b) 2007–09 (recent). We hypothesized that effective population size and genetic diversity would increase over time and that these management strategies would affect the resulting population genetic structure. We found trends indicating that the effective population size at the translocation site increased while that at the reintroduction sites diminished over time. The inbreeding coefficient of the translocated population diminished over time (from 0.38 to 0.03) and was much lower than that of the native (0.29) and reintroduced (0.13) recent populations. We observed a greater genetic admixture among the reintroduced sites on the historic sampling, as well as a strong genetic structure at the translocation site. In the recent sampling, the population structuring became more site-related suggesting low or inconsistent gene flow between sampling sites. This research highlights how conservation management decisions have an important influence on the genetic outcome of translocations and reintroductions. Future conservation planning should consider population genetic monitoring before and after management measures and maintain population connectivity thereafter to avoid the negative effects of a population size reduction.

(Source: S.O.S. Mata Atlântica)



Similar content being viewed by others
References
Allendorf FW (1986) Genetic drift and the loss of alleles versus heterozygosity. Zoo Biol 5:181–190. doi:10.1002/zoo.1430050212
Anderson EC, Dunham KK (2008) The influence of family groups on inferences made with the program structure. Mol Ecol Resour 8:1219–1229. doi:10.1111/j.1755-0998.2008.02355.x
Baker AJ, Dietz JM (1996) Immigration in wild groups of golden lion tamarins (Leontopithecus rosalia). Am J Primatol 38:47–56
Baker AJ, Dietz JM, Kleiman DG (1993) Behavioural evidence for monopolization of paternity in multi-male groups of golden lion tamarins. Anim Behav 46:1091–1103. doi:10.1006/anbe.1993.1299
Baker AJ, Bales K, Dietz J (2002) Mating system and group dynamics in lion tamarins. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Inst press, Washington, DC, pp 188–212
Ballou JD, Cooper A (1992) Genetic management strategies for endangered captive populations: the role of genetic and reproductive technology. Symp Zool Soc Lond 64:183–206
Ballou JD, Foose T (2010) Demographic and genetic management of captive populations. In: Kleiman DG, Thompson KV, Baer CK (eds) Wild mammals in captivity. Principles and techniques for zoo management. University of Chicago Press, USA, pp 263–283
Ballou JD, Kleiman DG, Mallinson JJC et al (2002) History, management, and conservation role of the captive lion tamarin populations. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Inst press, Washington, DC, pp 95–116
Beck BB, Castro MI (1994) Environments for endangered primates. In: Giubbons EF, Wyers E, Waters E, Menzel E (eds) Naturalistic environments in captivity for animal behavior research. State University of New York, Albany, pp 209–270
Beck BB, Kleiman DG, Dietz JM et al (1991) Losses and reproduction in reintroduced golden lion tamarins, Leontopithecus rosalia. Dodo 27:50–61
Beck BB, Castro MI, Stoinski TS, Ballou JD (2002) The effects of prerelease environments and postrelease management on survivorship in reintroduced golden lion tamarins. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Inst press, Washington, DC, pp 188–212
Bonin A, Bellemain E, Eidesen PB et al (2004) How to track and assess genotyping errors in population genetics studies. Mol Ecol 13:3261–3273. doi:10.1111/j.1365-294X.2004.02346.x
Bouzat JL, Johnson JA, Toepfer JE et al (2009) Beyond the beneficial effects of translocations as an effective tool for the genetic restoration of isolated populations. Conserv Genet 10:191–201. doi:10.1007/s10592-008-9547-8
Brekke P, Bennett PM, Santure AW, Ewen JG (2011) High genetic diversity in the remnant island population of hihi and the genetic consequences of re-introduction. Mol Ecol 20:29–45. doi:10.1111/j.1365-294X.2010.04923.x
Bristol RM, Tucker R, Dawson DA et al (2013) Comparison of historical bottleneck effects and genetic consequences of re-introduction in a critically endangered island passerine. Mol Ecol 22:4644–4662. doi:10.1111/mec.12429
Cain CM, Livieri TM, Swanson BJ (2011) Genetic evaluation of a reintroduced population of black-footed ferrets (Mustela nigripes). J Mammal 92:751–759. doi:10.1644/10-MAMM-S-104.1
Carlsson J (2008) Effects of microsatellite null alleles on assignment testing. J Hered 99:616–623. doi:10.1093/jhered/esn048
Coimbra-Filho AF, Mittermeier RA (1977) Conservation of the Brazilian lion tamarins Leontopithecus rosalia. In: HSH Prince Rainier III of Monaco & Bourne G (eds) Primate conservation. Academic Press, London, pp 59–94
Cullingham CI, Moehrenschlager A (2013) Temporal analysis of genetic structure to assess population dynamics of reintroduced swift foxes. Conserv Biol 27:1389–1398. doi:10.1111/cobi.12122
De Barba M, Waits LP, Garton EO et al (2010) The power of genetic monitoring for studying demography, ecology and genetics of a reintroduced brown bear population. Mol Ecol 19:3938–3951. doi:10.1111/j.1365-294X.2010.04791.x
Dietz JM, Baker AJ (1993) Polygyny and female reproductive success in golden lion tamarins, Leontopithecus rosalia. Anim Behav 46:1067–1078
Dietz JM, Baker AJ, Miglioretti D (1994) Seasonal variation in reproduction, juvenile growth, and adult body mass in golden lion tamarins (Leontopithecus rosalia). Am J Primatol 34:115–132. doi:10.1002/ajp.1350340204
Do C, Waples RS, Peel D et al (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214. doi:10.1111/1755-0998.12157
Dobson A, Ralls K, Foster M et al (1999) Corridors: reconnecting fragmented landscapes. In: Soulé ME, Terborgh J (eds) Continental conservation: scientific foundations of regional reserve networks. Island Press, Washington, DC, pp 129–170
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361. doi:10.1007/s12686-011-9548-7
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620. doi:10.1111/j.1365-294X.2005.02553.x
Fischer J, Lindenmayer DB (2000) An assessment of the published results of animal relocations. Biol Conserv 96:1–11. doi:10.1016/S0006-3207(00)00048-3
Frankham R (1995) Effective population size/adult population size ratios in wildlife: a review. Genet Res 66:95–107. doi:10.1017/S0016672308009695
Frankham R, Ballou J, Briscoe DA (2010) Introduction to conservation genetics, 2nd edn. Cambridge University Press, New York
Franklin IR, Frankham R (1998) How large must populations be to retain evolutionary potential? Anim Conserv 1:69–70
Galbusera PHA, Gillemot S (2008) Polymorphic microsatellite markers for the endangered golden-headed lion tamarin, Leontopithecus chrysomelas (Callitrichidae). Conserv Genet 9:731–733. doi:10.1007/s10592-007-9370-7
Goudet J (2005) HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol Ecol Notes 2:184–186. doi:10.1111/j.1471-8278.2004.00828.x
Grativol AD (2003) DNA antigo e genética da conservação do mico-leão-dourado (Leontopithecus rosalia): estrutura genética em duas escalas de tempo e sua relação com a fragmentação da Mata Atlântica. Acad. Thesis, Universidade Estadual do Norte Fluminense
Grativol AD, Ballou JD, Fleischer RC (2001) Microsatellite variation within and among recently isolated populations of golden lion tamarins (Leontopithecus rosalia). Conserv Genet 2:1–9
Griffith B, Scott J, Carpenter J, Reed C (1989) Translocation as a species conservation tool: status an strategy. Science 245:477–480
Guedes-Bruni RR, Silva Neto SJ da, Morim MP, Mantovani W (2006) Composição florística e estrutura de dossel em trecho de Floresta Ombrófila Densa Atlântica sobre morrote mamelonar na Reserva Biológica de Poço das Antas, Silva Jardim, Rio de Janeiro, Brazil. Rodriguésia 57:429–442
Habel JC, Husemann M, Finger A et al (2014) The relevance of time series in molecular ecology and conservation biology. Biol Rev 89:484–492. doi:10.1111/brv.12068
Holst B, Medici E, Marinho-Filho O et al (2006) Lion tamarin population and habitat viability assessment workshop2005: final report. Apple Valley, MN, USA
ICMBio (2016) Unidades de Conservação. http://www.icmbio.gov.br/portal/unidades-de-conservacao.html. Accessed 15 Jan 2016
IUCN (1987) The IUCN position statement on translocation of living organisms: introductions, re-introductions and re-stocking. https://portals.iucn.org/library/node/6507. Accessed 30 Sep 2016
IUCN/SSC (2013) Guidelines for reintroductions and other conservation translocations. Version 1.0. Gland, IUCN Species Survival Commission, Switzerland
Jorde PE, Ryman N (2007) Unbiased estimator for genetic drift and effective population size. Genetics 177:927–935. doi:10.1534/genetics.107.075481
Kalinowski ST (2005) HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5:187–189. doi:10.1111/j.1471-8286.2004.00845.x
Kalinowski ST (2011) The computer program STRUCTURE does not reliably identify the main genetic clusters within species: simulations and implications for human population structure. Heredity 106:625–632. doi:10.1038/hdy.2010.95
Kamath PL, Haroldson MA, Luikart G et al (2015) Multiple estimates of effective population size for monitoring a long-lived vertebrate: an application to Yellowstone grizzly bears. Mol Ecol 24:5507–5521. doi:10.1111/mec.13398
Kennington WJ, Hevroy TH, Johnson MS (2012) Long-term genetic monitoring reveals contrasting changes in the genetic composition of newly established populations of the intertidal snail Bembicium vittatum. Mol Ecol 21:3489–3500. doi:10.1111/j.1365-294X.2012.05636.x
Kierulff MCM (2000) Ecology and Behavior of translocated groups of golden lion tamarin (Leontopitehcus rosalia). Acad. Thesis, University of Cambridge
Kierulff MCM, Procópio-de-Oliveira P (1996) Re-assessing the status and conservation of the golden lion tamarin (Leontopithecus rosalia) in wild. Dodo J Jersey Wildl Preservation Trust 32:98–115
Kierulff MCM, Rylands AB (2003) Census and distribution of the golden lion tamarin (Leontopithecus rosalia). Am J Primatol 59:29–44. doi:10.1002/ajp.10064
Kierulff MCM, Procópio-de-Oliveira PP, Beck BB et al (2002) Reintroduction and translocation as conservation tools for golden lion tamarins. In: Kleiman DG, Rylands AB (eds) Lion tamarins: biology and conservation. Smithsonian Inst press, Washington, DC, pp 271–282
Kierulff MCM, Rylands AB, Procópio-de-Oliveira MM (2008) Leontopithecus rosalia. The IUCN Red List of Threatened Species 2008: e T11506A3287321. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T11506A3287321.en. Acessed 07 March 2016
Kierulff MCM, Ruiz-Miranda CR, Procópio-de-Oliveira P et al (2012) The Golden lion tamarin Leontopithecus rosalia: a conservation success story. Int Zoo Yearb 46:36–45. doi:10.1111/j.1748-1090.2012.00170.x
Kleiman D (1989) Reintroduction of captive mammals for conservation. Bioscience 39:152–161. doi:10.2307/1311025
Kleiman DG, Beck BB, Dietz JM et al (1986) Conservation program for the golden lion tamarin: captive research and management, ecological studies, educational strategies, and reintroduction. In: Benirhke K (ed) Primates. Springer-Verlag New York Inc., New York, pp 959–979
Kuo CH, Janzen FJ (2003) BOTTLESIM: a bottleneck simulation program for long-lived species with overlapping generations. Mol Ecol Notes 3:669–673. doi:10.1046/j.1471-8286.2003.00532.x
Maruyama T, Fuerst PA (1985) Population bottlenecks and nonequilibrium models in population genetics. II. Number of alleles in a small population that was formed by a recent bottleneck. Genetics 111:675–689
Meirmans PG (2015) Seven common mistakes in population genetics and how to avoid them. Mol Ecol 24:3223–3231. doi:10.1111/mec.13243
Michaelides S, Cole N, Funk SM (2015) Translocation retains genetic diversity of a threatened endemic reptile in Mauritius. Conserv Genet 16:661–672. doi:10.1007/s10592-014-0691-z
Mickelberg JL (2011) Understanding and managing isolation in a fragmented population of golden lion tamarins, Leontopithecus rosalia. Dissertation, George Mason University
Mock KE, Latch EK, Rhodes OE (2004) Assessing losses of genetic diversity due to translocation: Long-term case histories in Merriam’s turkey (Meleagris gallopavo merriami). Conserv Genet 5:631–645. doi:10.1007/s10592-004-1849-x
Mowry RA, Schneider TM, Latch EK et al (2015) Genetics and the successful reintroduction of the Missouri river otter. Anim Conserv 18:196–206. doi:10.1111/acv.12159
Ortego J, Yannic G, Shafer ABA et al (2011) Temporal dynamics of genetic variability in a mountain goat (Oreamnos americanus) population. Mol Ecol 20:1601–1611. doi:10.1111/j.1365-294X.2011.05022.x
Parker KA (2008) Translocations: providing outcomes for wildlife, resource managers, scientists, and the human community. Restor Ecol 16:204–209. doi:10.1111/j.1526-100X.2008.00388.x
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. doi:10.1111/j.1471-8286.2005.01155.x
Perez-Sweeney BM, Valladares-Padua C, Burrell AS et al (2005) Dinucleotide microsatellite primers designed for a critically endangered primate, the black lion tamarin (Leontopithecus chrysopygus). Mol Ecol Notes 5:198–201. doi:10.1111/j.1471-8286.2005.00875.x
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959. doi:10.1111/j.1471-8286.2007.01758.x
Procópio-de-Oliveira P, Grativol A, Ruiz-Miranda C (2008) Conservação do mico-leão-dourado: enfrentando os desafios de uma paisagem fragmentada. Universidade Estadual do Norte Fluminense Darcy Ribeiro press, Campos dos Goytacazes, RJ, Brazil
R Development Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ribeiro MC, Metzger JP, Martensen AC et al (2009) The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153. doi:10.1016/j.biocon.2009.02.021
Rice WR (1989) Analyzing tables of statistical tests. Evolution Int J Org Evolution 43:223–225
Sasmal I, Jenks JA, Waits LP et al (2013) Genetic diversity in a reintroduced swift fox population. Conserv Genet 14:93–102. doi:10.1007/s10592-012-0429-8
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Schwartz MK, McKelvey KS (2009) Why sampling scheme matters: the effect of sampling scheme on landscape genetic results. Conserv Genet 10:441–452. doi:10.1007/s10592-008-9622-1
Seddon PJ, Armstrong DP, Maloney RF (2007) Developing the science of reintroduction biology. Conserv Biol 21:303–312. doi:10.1111/j.1523-1739.2006.00627.x
Sigg DP, Goldizen AW, Pople AR (2005) The importance of mating system in translocation programs: reproductive success of released male bridled nailtail wallabies. Biol Conserv 123:289–300. doi:10.1016/j.biocon.2004.11.017
Sinnock P (1975) The wahlund effect for the two-locus model. Am Nat 109:565–569. doi:10.2307/2678832
Stephen C, Whittaker D, Gillis D et al (2005) Genetic consequences of reintroductions: an example from oregon pronghorn antelope (Antilocapra americana). J Wildl Manage 69:1463–1474. doi:10.2193/0022-541X(2005)69[1463:GCORAE]2.0.CO;2
Stoinski TS, Beck BB, Bowman MD, Lenhardt J (1997) The gateway zoo program: a recent initiative in golden lion tamarin reintroductions. In: Wallis J (ed) Primate conservation: the role of zoological parks. American Society of Primatologists, Norman, OK, pp 113–130
Thrimawithana AH, Ortiz-Catedral L, Rodrigo A, Hauber ME (2013) Reduced total genetic diversity following translocations? A metapopulation approach. Conserv Genet 14:1043–1055. doi:10.1007/s10592-013-0494-7
Tollington S, Jones CG, Greenwood A et al (2013) Long-term, fine-scale temporal patterns of genetic diversity in the restored Mauritius parakeet reveal genetic impacts of management and associated demographic effects on reintroduction programmes. Biol Conserv 161:28–38. doi:10.1016/j.biocon.2013.02.013
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538. doi:10.1111/j.1471-8286.2004.00684.x
Wang J (2005) Estimation of effective population sizes from data on genetic markers. Philos Trans R Soc Lond B Biol Sci 360:1395–1409. doi:10.1098/rstb.2005.1682
Waples RS (2005) Genetic estimates of contemporary effective population size: to what time periods do the estimates apply? Mol Ecol 14:3335–3352. doi:10.1111/j.1365-294X.2005.02673.x
Waples RS (2006) A bias correction for estimates of effective population size based on linkage disequilibrium at unlinked gene loci. Conserv Genet 7:167–184. doi:10.1007/s10592-005-9100-y
Waples RS, Do C (2008) LDNE: a program for estimating effective population size from data on linkage disequilibrium. Mol Ecol Resour 8:753–756. doi:10.1111/j.1755-0998.2007.02061.x
Waples RS, Do C (2010) Linkage disequilibrium estimates of contemporary Ne using highly variable genetic markers: a largely untapped resource for applied conservation and evolution. Evol Appl 3:244–262. doi:10.1111/j.1752-4571.2009.00104.x
Acknowledgements
We are grateful for support by the LTBF and by K. De Vleeschouwer and P. Galbusera of the Centre for Research and Conservation funded by the Flemish Government. CNPq supported PMG Jr (308385/2014-4), MCR (312045/2013-1) and CRRM (472647/2009-1); and FAPESP supports MCR (2013/50421-2). Scholarship received by AMM (CAPES), CSC (FAPESP 2014/01029-5) and MCMK (PNPD/CAPES). We thank M. King, a native English speaker, for proofreading the manuscript; A. Nicodemo for assistance in the laboratory; and AMLD, especially A. Martins and P. Procópio-de-Oliveira, for providing the field data and hair samples. University of Maryland Animal Care and Use Committee approved protocols for this research. The authors thank the three anonymous reviewers for suggestions and comments, which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moraes, A.M., Ruiz-Miranda, C.R., Ribeiro, M.C. et al. Temporal genetic dynamics of reintroduced and translocated populations of the endangered golden lion tamarin (Leontopithecus rosalia). Conserv Genet 18, 995–1009 (2017). https://doi.org/10.1007/s10592-017-0948-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-017-0948-4