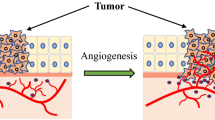
Abstract
Combretastatin can prevent the supply of nutrients to cancer cells by selectively interrupting tumor blood flow (TBF). Therefore, combretastatin may serve as a new anticancer drug that utilizes starvation tactics to attack solid tumors. Among combretastatin compounds, combretastatin A-4 and a combretastatin A-4 derivative (Cderiv) are now in phase III clinical trials. These two combretastatin compounds have similar chemical structures and provide marked TBF interruption. However, their mechanisms of action are reportedly quite different and remain controversial. Precise mechanisms of action of these agents must be elucidated so as to develop safe clinical treatments and wider clinical applications. By using various kinds of rodent tumors, we showed that Cderiv produced potent interruption of TBF in all primary tumors and metastatic foci, without exception, and had beneficial therapeutic effects including significantly improved survival. Cderiv caused host arterioles to constrict. However, a tumor vascular bed scarcely reacted to a direct topical application of Cderiv. In addition, the fact that Cderiv did not have cytotoxic drug-like accumulated toxicity usually caused by repeated administration means that inhibition of tubulin polymerization by Cderiv may not occur to a great degree in vivo. Therefore, at least for Cderiv, our studies demonstrated that TBF interruption was mainly caused indirectly, via enhancement of vascular resistance of host arterioles, rather than being caused by a direct effect of Cderiv on tumor vessels. In this review, I describe cancer therapy that utilizes such TBF interruption, which leads to Cderiv-induced necrosis, and discuss details of its microcirculation mechanism.














Similar content being viewed by others
References
Schlaeppi, J. M., & Wood, J. M. (1999). Targeting vascular endothelial growth factor (VEGF) for anti-tumor therapy, by anti-VEGF neutralizing monoclonal antibodies or by VEGF receptor tyrosine-kinase inhibitors. Cancer and Metastasis Review, 18, 473–481.
Folkman, J. (2003). Angiogenesis inhibitors: a new class of drugs. Cancer Biology & Therapy, 2, S127–S133.
Abdollahi, A., & Folkman, J. (2010). Evading tumor evasion: Current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resistance Updates, 13, 16–28.
Korpanty, G., Smyth, E., & Carney, D. N. (2011). Update on anti-angiogenic therapy in non-small cell lung cancer: Are we making progress? Journal of Thoracic Disease, 3, 19–29.
Tozer, G. M., Kanthou, C., & Baguley, B. C. (2005). Disrupting tumour blood vessels. Nature Reviews. Cancer, 5, 423–435.
Hori, K. (2005). Cancer therapy by means of irreversible tumor blood flow stasis: Starvation tactics against solid tumors. Gene Therapy and Molecular Biology, 9, 203–216.
Kanthou, C., & Tozer, G. M. (2009). Microtubule depolymerizing vascular disrupting agents: Novel therapeutic agents for oncology and other pathologies. International Journal of Experimental Pathology, 90, 284–294.
McKeage, M. J., & Baguley, B. C. (2010). Disrupting established tumor blood vessels: An emerging therapeutic strategy for cancer. Cancer, 116, 1859–1871.
Siemann, D. W. (2011). The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treatment Reviews, 37, 63–74.
Folkman, J. (1971). Tumor angiogenesis: Therapeutic implications. New England Journal of Medicine, 285, 1182–1186.
Ferrara, N. (2002). VEGF and the quest for tumour angiogenesis factors. Nature Review Cancer, 2, 795–803.
Ferrara, N., & Kerbel, R. S. (2005). Angiogenesis as a therapeutic target. Nature, 438, 967–974.
Willett, C. G., Boucher, Y., di Tomaso, E., Duda, D. G., Munn, L. L., Tong, R. T., et al. (2004). Direct evidence that the VEGF specific antibody bevacizumab has antivascular effects in human rectal cancer. Nature Medicine, 10, 145–147.
Ricci-Vitiani, L., Pallini, R., Biffoni, M., Todaro, M., Invernici, G., Cenci, T., et al. (2010). Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature, 468, 824–828.
Wang, R., Chadalavada, K., Wilshire, J., Kowalik, U., Hovinga, K. E., Geber, A., et al. (2010). Glioblastoma stem-like cells give rise to tumour endothelium. Nature, 468, 829–833.
Soda, Y., Marumoto, T., Friedmann-Morvinski, D., Soda, M., Liu, F., Michiue, H., et al. (2011). Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proceedings of the National Academy of Sciences of the United States of America, 108, 4274–4280.
Warren, B. A., & Shubik, P. (1966). The growth of the blood supply to melanoma transplants in the hamster cheek pouch. Laboratory Investigation, 15, 464–478.
Hammersen, F., Endrich, B., & Messmer, K. (1985). The fine structure of tumor blood vessels. I. participation of non-endothelial cells in tumor angiogenesis. International Journal of Microcirculation Clinical and Experimental, 4, 31–43.
Maniotis, A. J., Folberg, R., Hess, A., Seftor, E. A., Gardner, L. M., Pe’er, J., et al. (1999). Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. American Journal of Pathology, 155, 739–752.
Carmeliet, P., & Jain, R. K. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature, 473, 298–307.
Ohsumi, K., Hatanaka, T., Fujita, K., Nakagawa, R., Fukuda, Y., Nihei, Y., et al. (1998). Syntheses and antitumor activity of cis-restricted combretastatins: 5-Membered heterocyclic analogues. Bioorganic & Medical Chemistry Letters, 8, 3153–3158.
Goldstein, H. M., Wallace, S., Anderson, J. H., Bree, R. L., & Gianturco, C. (1976). Transcatheter occlusion of abdominal tumors. Radiology, 120, 539–545.
Chung, V. P., & Wallace, S. (1981). Hepatic artery embolization in the treatment of hepatic neoplasms. Radiology, 140, 51–58.
Denekamp, J., Hill, S. A., & Hobson, B. (1983). Vascular occlusion and tumour cell death. European Journal of Cancer & Clinical Oncology, 19, 271–275.
Kelly, M. G., & Hartwell, J. L. (1954). The biological effects and the chemical composition of podophyllin: A review. Journal of the National Cancer Institute, 14, 967–1010.
Sackett, D. L. (1993). Podophyllotoxin, steganacin and combretastatin: Natural products that bind at the colchicine site of tubulin. Pharmacology & Therapeutics, 59, 163–228.
Algire, G. H., Legallais, F. Y., & Anderson, B. F. (1954). Vascular reactions of normal and malignant tissues in vivo. VI. The role of hypotension in the action of components of podophyllin on transplanted sarcomas. Journal of the National Cancer Institute, 14, 879–893.
Yue, Q.-X., Liu, X., & Guo, D.-A. (2010). Microtubule-binding natural products for cancer therapy. Planta Medica, 76, 1037–1043.
Hill, S. A., Lonergan, S. J., Denekamp, J., & Chaplin, D. J. (1993). Vinca alkaloids: anti-vascular effects in a murine tumour. European Journal of Cancer, 29, 1320–1324.
Pettit, G. R., Cragg, G. M., Herald, D. L., Schmidt, J. M., & Lohavanijaya, P. (1982). Isolation and structure of combretastatin. Canadian Journal of Chemistry, 60, 1374–1376.
Pettit, G. R., Singh, S. B., Hamel, E., Lin, C. M., Alberts, D. S., & Garcia-Kendall, D. (1989). Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia, 15, 209–211.
Lin, C. M., Ho, H. H., Pettit, G. R., & Hamel, E. (1989). Antimitotic natural products combretastatin A-4 and combretastatin A-2: Studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry, 28, 6984–6991.
Dark, G. G., Hill, S. A., Prise, V. E., Tozer, G. M., Pettit, G. R., & Chaplin, D. J. (1997). Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Research, 57, 1829–1834.
Nam, N. H. (2003). Combretastatin A-4 analogues as antimitotic antitumor agents. Current Medicinal Chemistry, 10, 1697–1722.
Monk, K. A., Siles, R., Hadimani, M. B., Mugabe, B. E., Ackley, J. F., Studerus, S. W., et al. (2006). Design, synthesis, and biological evaluation of combretastatin nitrogen-containing derivatives as inhibitors of tubulin assembly and vascular disrupting agents. Bioorganic & Medicinal Chemistry, 14, 3231–3244.
Dupeyre, G., Chabot, G. G., Thoret, S., Cachet, X., Seguin, J., Guénard, D., et al. (2006). Synthesis and biological evaluation of (3,4,5-trimethoxyphenyl)indol-3-ylmethane derivatives as potential antivascular agents. Bioorganic & Medicinal Chemistry, 14, 4410–4426.
Hori, K. (2010). Cancer relapse prevention by means of tumor blood flow interruption after radiotherapy. Hoshasen Seibutsu Kenkyu, 45, 213–227.
Nihei, Y., Suga, Y., Morinaga, Y., Ohishi, K., Okano, A., Ohsumi, K., et al. (1999). A novel combretastatin A-4 derivative, AC-7700, shows marked antitumor activity against advanced solid tumors and orthotopically transplanted tumors. Japanese Journal of Cancer Research, 90, 1016–1025.
Hori, K., Saito, S., Nihei, Y., Suzuki, M., & Sato, Y. (1999). Antitumor effects due to irreversible stoppage of tumor tissue blood flow: Evaluation of a novel combretastatin A-4 derivative, AC7700. Japanese Journal of Cancer Research, 90, 1026–1038.
Nihei, Y., Suzuki, M., Okano, A., Tsuji, T., Akiyama, Y., Tsuruo, T., et al. (1999). Evaluation of antivascular and antimitotic effects of tubulin binding agents in solid tumor therapy. Japanese Journal of Cancer Research, 90, 1387–1395.
Hori, K., Saito, S., Sato, Y., & Kubota, K. (2001). Stoppage of blood flow in 3-methylcholanthrene-induced autochthonous primary tumor due to a novel combretastatin A-4 derivative, AC7700, and its antitumor effect. Medical Science Monitor, 7, 26–33.
Hori, K., Saito, S., & Kubota, K. (2002). A novel combretastatin A-4 derivative, AC7700, strongly stanches tumour blood flow and inhibits growth of tumours developing in various tissues and organs. British Journal of Cancer, 86, 1604–1614.
Hori, K., Saito, S., Sato, Y., Akita, H., Kawaguchi, T., Sugiyama, K., et al. (2003). Differential relationship between changes in tumour size and microcirculatory functions induced by therapy with an antivascular drug and with cytotoxic drugs: implications for evaluation of therapeutic efficacy of AC7700 (AVE8062). European Journal of Cancer, 39, 1957–1966.
Hori, K., & Saito, S. (2003). Microvascular mechanisms by which the combretastatin A-4 derivative AC7700 (AVE8062) induces tumor blood flow stanching. British Journal of Cancer, 89, 1334–1344.
Hori, K., & Saito, S. (2004). Induction of tumour blood flow stasis and necrosis: A new function for epinephrine similar to that of combretastatin A-4 derivative AVE8062 (AC7700). British Journal of Cancer, 90, 549–553.
Ohno, T., Kawano, K., Sasaki, A., Aramaki, M., Tahara, K., Etoh, T., et al. (2002). Antitumor and antivascular effects of AC-7700, a combretastatin A-4 derivative, against rat liver cancer. International Journal of Clinical Oncology, 7, 171–176.
Tozer, G. M., Prise, V. E., Wilson, J., Cemazar, M., Shan, S., Dewhirst, M. W., et al. (2001). Mechanisms associated with tumor vascular shut-down induced by combretastatin A-4 phosphate: Intravital microscopy and measurement of vascular permeability. Cancer Research, 61, 6413–6422.
Eikesdal, H. P., Landuyt, W., & Dahl, O. (2002). The influence of combretastatin A-4 and vinblastine on interstitial fluid pressure in BT4An rat gliomas. Cancer Letters, 178, 209–217.
Ley, C. D., Horsman, M. R., & Kristjansen, P. E. (2007). Early effects of combretastatin-A4 disodium phosphate on tumor perfusion and interstitial fluid pressure. Neoplasia, 9, 108–112.
Kanthou, C., & Tozer, G. M. (2002). The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood, 99, 2060–2069.
Nallamothu, R., Wood, G. C., Kiani, M. F., Moore, B. M., Horton, F. P., & Thoma, L. A. (2006). A targeted liposome delivery system for combretastatin A4: Formulation optimization through drug loading and in vitro release studies. PDA Journal of Pharmaceutical Science and Technology, 60, 144–155.
Wang, Y., Yang, T., Wang, X., Wang, J., Zhang, X., & Zhang, Q. (2010). Targeted polymeric micelle system for delivery of combretastatin A4 to tumor vasculature in vitro. Pharmaceutical Research, 27, 1861–1868.
Hori, K., Zhang, Q.-H., Saito, S., Tanda, S., Li, H.-C., & Suzuki, M. (1993). Microvascular mechanisms of change in tumor blood flow due to angiotensin II, epinephrine, and methoxamine: A functional morphometric study. Cancer Research, 53, 5528–5534.
Hori, K., Suzuki, M., Tanda, S., & Saito, S. (1990). In vivo analysis of tumor vascularization in the rat. Japanese Journal of Cancer Research, 81, 279–288.
Nicoll, P. A., & Webb, R. L. (1946). Blood circulation in the subcutaneous tissue of the living bat’s wing. Annals of the New York Academy of Sciences, 46, 697–711.
Lutz, B. R., & Fulton, G. P. (1954). The use of the hamster cheek pouch for the study of vascular changes at the microscopic level. The Anatomical Record, 120, 293–307.
Strahler, A. N. (1957). Quantitative analysis of watershed geomorphology. Transactions American Geophysical Union, 38, 913–920.
Kruuv, J. A., Inch, W. R., & McCredie, J. A. (1967). Blood flow and oxygenation of tumors in mice. II. Effects of vasodilator drugs. Cancer, 20, 60–65.
Suzuki, M., Hori, K., Abe, I., Saito, S., & Sato, H. (1984). Functional characterization of the microcirculation in tumors. Cancer Metastasis Review, 3, 115–126.
Jirtle, R. L. (1988). Chemical modification of tumour blood flow. International Journal of Hyperthermia, 4, 355–371.
Hori, K., & Suzuki, M. (1992). Metastasis and vascularization. Mebio, 9, 50–56.
Hori, K., Suzuki, M., Tanda, S., & Saito, S. (1991). Characterization of heterogeneous distribution of tumor blood flow in the rat. Japanese Journal of Cancer Research, 82, 109–117.
Suzuki, M., Hori, K., Abe, I., Saito, S., & Sato, H. (1981). A new approach to cancer chemotherapy: Selective enhancement of tumor blood flow with angiotensin II. Journal of the National Cancer Institute, 67, 663–669.
Hori, K. (2003). Cancer therapy due to irreversible tumor blood flow stanching: A novel therapeutic strategy for refractory cancers. Kareiigaku Kenkyusho Zasshi, 53, 1–19.
Acknowledgments
I am grateful to Dr. Sachiko Saito for helpful suggestions and to Ms. Hiroko Oikawa for assistance with experiments. This study was supported by Grant-in-Aid for Cancer Research (12-Designated Research-1) from the Ministry of Health, Labor and Welfare, Japan; by Grant-in-Aid (No. 13218010; No. 14030005; No. 17591242) for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan; and by the Haruo Sato Fund for Yoshida Sarcoma and Ascites Hepatoma Memorial.
Conflicts of interest
I declare that I have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hori, K. Starvation tactics for solid tumors: tumor blood flow interruption via a combretastatin derivative (Cderiv), and its microcirculation mechanism. Cancer Metastasis Rev 31, 109–122 (2012). https://doi.org/10.1007/s10555-011-9333-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9333-9