Abstract
Purpose
Women with HER2-positive breast cancer treated prior to effective anti-HER2 therapy have higher rates of local–regional recurrence (LRR) than those with HER2-negative disease. Effective systemic therapy, however, has been shown to decrease LRR. This study examines LRR in women with HER2-positive breast cancer treated on a single-arm prospective multicenter trial of adjuvant trastuzumab (H) and paclitaxel (T).
Methods
Patients with HER2-positive tumors ≤ 3.0 cm with negative axillary nodes or micrometastatic disease were eligible. Systemic therapy included weekly T and H for 12 weeks followed by continuation of H to complete 1 year. Radiation therapy (RT) was required following breast-conserving surgery (BCS), but dose and fields were not specified. Disease-free survival (DFS) and LRR-free survival were calculated using the Kaplan–Meier method.
Results
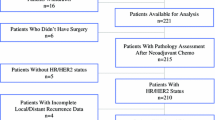
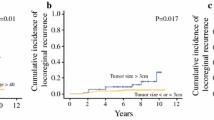
Of the 410 patients enrolled from September 2007 to September 2010, 406 initiated protocol therapy and formed the basis of this analysis. A total of 272 (67%) had hormone receptor-positive tumors. Of 162 patients undergoing mastectomy, local therapy records were unavailable for two. None of the 160 for whom records were available received RT. Among 244 BCS patients, detailed RT records were available for 217 (89%). With a median follow-up of 6.5 years, 7-year DFS was 93.3% (95% CI 90.4–96.2), and LRR-free survival was 98.6% (95% CI 97.4–99.8).
Conclusion
LRR in this select group of early-stage patients with HER2-positive disease receiving effective anti-HER2 therapy is extremely low. If confirmed in additional studies, future investigational efforts should focus on de-escalating local therapy.


Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100(14):8418–8423. https://doi.org/10.1073/pnas.0932692100
Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL, Harris JR (2008) Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 26(14):2373–2378. https://doi.org/10.1200/JCO.2007.14.4287
Millar EK, Graham PH, O’Toole SA, McNeil CM, Browne L, Morey AL, Eggleton S, Beretov J, Theocharous C, Capp A, Nasser E, Kearsley JH, Delaney G, Papadatos G, Fox C, Sutherland RL (2009) Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol 27(28):4701–4708. https://doi.org/10.1200/JCO.2008.21.7075
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28(10):1684–1691. https://doi.org/10.1200/JCO.2009.24.9284
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0
Sartor CI, Peterson BL, Woolf S, Fitzgerald TJ, Laurie F, Turrisi AJ, Bogart J, Henderson IC, Norton L (2005) Effect of addition of adjuvant paclitaxel on radiotherapy delivery and locoregional control of node-positive breast cancer: cancer and leukemia group B 9344. J Clin Oncol 23(1):30–40. https://doi.org/10.1200/JCO.2005.12.044
Anderson SJ, Wapnir I, Dignam JJ, Fisher B, Mamounas EP, Jeong JH, Geyer CE Jr, Wickerham DL, Costantino JP, Wolmark N (2009) Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol 27(15):2466–2473. https://doi.org/10.1200/JCO.2008.19.8424
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. https://doi.org/10.1056/NEJMoa052122
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I, Wolff AC, Carey LA, Overmoyer BA, Partridge AH, Guo H, Hudis CA, Krop IE, Burstein HJ, Winer EP (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2):134–141. https://doi.org/10.1056/NEJMoa1406281
Tseng YD, Uno H, Hughes ME, Niland JC, Wong YN, Theriault R, Blitzblau RC, Moy B, Breslin T, Edge SB, Hassett MJ, Punglia RS (2015) Biological subtype predicts risk of locoregional recurrence after mastectomy and impact of postmastectomy radiation in a large national database. Int J Radiat Oncol Biol Phys 93(3):622–630. https://doi.org/10.1016/j.ijrobp.2015.07.006
Yin W, Jiang Y, Shen Z, Shao Z, Lu J (2011) Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS ONE 6(6):e21030. https://doi.org/10.1371/journal.pone.0021030
Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, Weir LM, Olivotto IA (2004) Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med 351(10):963–970. https://doi.org/10.1056/NEJMoa040595
Liu FF, Shi W, Done SJ, Miller N, Pintilie M, Voduc D, Nielsen TO, Nofech-Mozes S, Chang MC, Whelan TJ, Weir LM, Olivotto IA, McCready DR, Fyles AW (2015) Identification of a low-risk luminal a breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol 33(18):2035–2040. https://doi.org/10.1200/JCO.2014.57.7999
Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, Vallis KA, White JR, Rousseau P, Fortin A, Pierce LJ, Manchul L, Chafe S, Nolan MC, Craighead P, Bowen J, McCready DR, Pritchard KI, Gelmon K, Murray Y, Chapman JA, Chen BE, Levine MN, Investigators MAS (2015) Regional nodal irradiation in early-stage breast cancer. N Engl J Med 373(4):307–316. https://doi.org/10.1056/NEJMoa1415340
Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, Collette L, Fourquet A, Maingon P, Valli M, De Winter K, Marnitz S, Barillot I, Scandolaro L, Vonk E, Rodenhuis C, Marsiglia H, Weidner N, van Tienhoven G, Glanzmann C, Kuten A, Arriagada R, Bartelink H, Van den Bogaert W, Oncology ER, Breast Cancer G (2015) Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 373(4):317–327. https://doi.org/10.1056/NEJMoa1415369
Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N (2002) Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 347(8):567–575. https://doi.org/10.1056/NEJMoa020128
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106. https://doi.org/10.1016/S0140-6736(05)67887-7
Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, Blichert-Toft M, Mouridsen HT (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353(9165):1641–1648. https://doi.org/10.1016/S0140-6736(98)09201-0
Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 337(14):949–955. https://doi.org/10.1056/NEJM199710023371401
Punglia RS, Morrow M, Winer EP, Harris JR (2007) Local therapy and survival in breast cancer. N Engl J Med 356(23):2399–2405. https://doi.org/10.1056/NEJMra065241
Funding
Genentech provided funding for the conduct of this trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JB receives honorarium from UpToDate, Wolters Kluwer, The International Journal of Radiation Oncology, Biology and Physics, Leidos Pharmaceuticals, Accuray, and research funding from Prosigna. WTB reports research funding (institution) from Pfizer. CTD has received institutional research funding from and has served as advisor/consultant for Roche/Genentech, PUMA, Pfizer, Amgen, GlaxoSmithKline; in last 2 years CTD has received institutional research funding from Roche/Genentech and PUMA. BM receives institutional research funding from Puma Biotechnology. KSA has received one-time advisory board honoraria (with travel reimbursements) from Genentech/Roche, Genomic Health Inc, Novartis, Pfizer and Myriad and from Puma (as chair of an IDMC). ACW receives institutional research funding from Biomarin, Celldex, and Pfizer.BAO receives institutional research funding from Eisai, Incyte. IEK receives institutional research funding from Genentech/Roche and Pfizer and served as an advisor/consultant and received honoraria from Genentech/Roche, Daiichi/Sankyo, Macrogenomics, Context Therapeutics, Seattle Genetics and Taiho Oncology. EPW has served as an advisor to Genentech/Roche, Eli Lilly, and GSK, and serves on the Scientific Advisory Board for Leap Therapeutics. SMT receives institutional research funding from Novartis, Genentech, Eli Lilly, Pfizer, Merck, Exelixis, Eisai, Bristol Meyers Squibb, AstraZeneca, Cyclacel, Immunomedics, Odenate, and Nektar. SMT has served as an advisor/consultant to Novartis, Eli Lilly, Pfizer, Merck, AstraZeneca, Eisai, Puma, Genentech, Immunomedics, Nektar, Tesaro, Bristol Meyers Squibb, and Nanostring.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellon, J.R., Guo, H., Barry, W.T. et al. Local–regional recurrence in women with small node-negative, HER2-positive breast cancer: results from a prospective multi-institutional study (the APT trial). Breast Cancer Res Treat 176, 303–310 (2019). https://doi.org/10.1007/s10549-019-05238-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05238-4