Abstract
Background
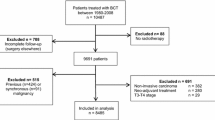
Increasingly, women with stage 2 and 3 breast cancers receive neoadjuvant therapy, after which many are eligible for breast-conserving surgery (BCS). The question often arises as to whether BCS, if achievable, provides adequate local control. We report the results of local recurrence (LR) from the I-SPY 1 Trial in the setting of maximal multidisciplinary treatment where approximately 50 % of patients were treated with BCS.
Methods
We analyzed data from the I-SPY 1 Trial. Women with tumors ≥3 cm from nine clinical breast centers received neoadjuvant doxorubicin, cyclophosphamide and paclitaxel followed by definitive surgical therapy, and radiation at physician discretion. LR following mastectomy and BCS were analyzed in relation to clinical characteristics and response to therapy as measured by residual cancer burden.
Results
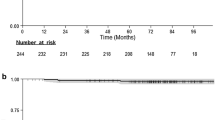
Of the 237 patients enrolled in the I-SPY 1 Trial, 206 were available for analysis. Median tumor size was 6.0 cm, and median follow-up was 3.9 years. Fourteen patients (7 %) had LR and 45 (22 %) had distant recurrence (DR). Of the 14 patients with LR, nine had synchronous DR; one had DR > 2 years later. Only four (2 % of evaluable patients) had LR alone. The rate of LR was low after mastectomy and after BCS, even in the setting of significant residual disease.
Conclusions
Overall, these patients at high risk for early recurrence, treated with maximal multidisciplinary treatment, had low LR. Recurrence was associated with aggressive biological features such as more advanced stage at presentation, where LR occurs most frequently in the setting of DR.



Similar content being viewed by others
References
Lin C, Buxton MB, Moore D, et al. Locally advanced breast cancers are more likely to present as interval cancers: results from the I-SPY 1 TRIAL (CALGB 150007/150012, ACRIN 6657, InterSPORE Trial). Breast Cancer Res Treat. 2012;132:871–79.
Rouzier R, Mathieu MC, Sideris L, et al. Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer. 2004;101:918–25.
Pierga JY, Mouret E, Laurence V, et al. Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer: the role of clinical response. Eur J Cancer. 2003;39:1089–96.
Esserman LJ, Moore DH, Tsing PJ, et al. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. 2011;129:607–16.
Esserman LJ, Berry DA, Demichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–49.
Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2012;132:1049–62.
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy [see comments]. J Clin Oncol. 1999;17:460–69.
Kuerer HM, Newman LA, Buzdar AU, et al. Residual metastatic axillary lymph nodes following neoadjuvant chemotherapy predict disease-free survival in patients with locally advanced breast cancer. Am J Surg. 1998;176:502–09.
Kuerer HM, Newman LA, Fornage BD, et al. Role of axillary lymph node dissection after tumor downstaging with induction chemotherapy for locally advanced breast cancer [see comments]. Ann Surg Oncol. 1998;5:673–80.
Kuerer HM, Newman LA, Buzdar AU, et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary lymph node status. Cancer J Sci Am. 1998;4:230–36.
Pierga JY, Mouret E, Dieras V, et al. Prognostic value of persistent node involvement after neoadjuvant chemotherapy in patients with operable breast cancer. Br J Cancer. 2000;83:1480–87.
Schwartz GF, Birchansky CA, Komarnicky LT, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73:362–69.
Cance WG, Carey LA, Calvo BF, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236:295–02;discussion 302–293.
Hylton NM, Blume J, Bernreuter W, et al. Prediction of response to neoadjuvant chemotherapy for women with locally-advanced breast cancer: results from the ACRIN 6657/ I-SPY Trial. Radiology. 2012;263:663–72.
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22.
Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson Cancer Center experience. J Clin Oncol. 2004;22:2303–12.
Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. Cancer. 2005;103:689–95.
Min SY, Lee SJ, Shin KH, et al. Locoregional recurrence of breast cancer in patients treated with breast conservation surgery and radiotherapy following neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2011;81:e697–05.
Rouzier R, Extra JM, Carton M, et al. Primary chemotherapy for operable breast cancer: incidence and prognostic significance of ipsilateral breast tumor recurrence after breast-conserving surgery. J Clin Oncol. 2001;19:3828–35.
Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol. 1999;10:47–52.
Beriwal S, Schwartz GF, Komarnicky L, Garcia-Young JA. Breast-conserving therapy after neoadjuvant chemotherapy: long-term results. Breast J. 2006;12:159–64.
Cebrecos I, Cordoba O, Deu J, et al. Can we predict local recurrence in breast conserving surgery after neoadjuvant chemotherapy? Eur J Surg Oncol. 2010;36:528–34.
Huang EH, Strom EA, Perkins GH, et al. Comparison of risk of local-regional recurrence after mastectomy or breast conservation therapy for patients treated with neoadjuvant chemotherapy and radiation stratified according to a prognostic index score. Int J Radiat Oncol Biol Phys. 2006;66:352–57.
Garg AK, Strom EA, McNeese MD, et al. T3 disease at presentation or pathologic involvement of four or more lymph nodes predict for locoregional recurrence in stage II breast cancer treated with neoadjuvant chemotherapy and mastectomy without radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:138–45.
Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:351–57.
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93.
Rouzier R, Pusztai L, Delaloge S, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23:8331–39.
Uematsu T, Kasami M, Watanabe J, et al. Is lymphovascular invasion degree one of the important factors to predict neoadjuvant chemotherapy efficacy in breast cancer? Breast Cancer. 2011;18:309–13.
Fasching PA, Heusinger K, Haeberle L, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486.
Gianni L, Zambetti M, Clark K, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23:7265–77.
Mukhtar RA, Yau C, Rosen M, et al. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol. 2013;20:3823–30.
Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–06.
Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;1:309–26.
Berry T, Brooks S, Sydow N, et al. Complication rates of radiation on tissue expander and autologous tissue breast reconstruction. Ann Surg Oncol. 2010;17 Suppl 3:202–10.
Brooks S, Djohan R, Tendulkar R, et al. Risk factors for complications of radiation therapy on tissue expander breast reconstructions. Breast J. 2012;18:28–34.
Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105:930–42.
Peled A, Foster R, Esserman L. A comparison of oncoplastic reduction mammoplasty and mastectomy with immediate reconstruction in patients with locally advanced breast cancer. 2012. In press.
Acknowledgement
We wish to thank the study patients and patient advocates. There are also several people who were the champions of this I-SPY Trial, without whom the study would not have been initiated: Jorge Gomez from the National Cancer Institute (NCI); Larry Norton from CALGB; and Ken Buetow and Subha Madhavan for enabling the development of the data systems for the trial data. We also wish to thank Rachel Gomez for developing the training and systems to guarantee the quality of the pathologic data, and to Sarah Davis for her efforts to ensure the integrity of the entire data set, and to Meredith Buxton for coordinating the entire study. We would also like to thank Lisa Carey from the University of North Carolina for her valuable input. Funding was received from the NCI SPORE in Breast Cancer (CA58207), ACRIN (CA079778 and CA080098), CALGB (CA31964 & CA33601), NCI Center for Bioinformatics, The Breast Cancer Research Foundation, Bruce and Martha Atwater, The Terry Fox Foundation Postdoctoral Fellowship, and ‘Give Breast Cancer the Boot’.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted on behalf of the I-SPY 1 TRIAL Investigators.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cureton, E.L., Yau, C., Alvarado, M.D. et al. Local Recurrence Rates are Low in High-Risk Neoadjuvant Breast Cancer in the I-SPY 1 Trial (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol 21, 2889–2896 (2014). https://doi.org/10.1245/s10434-014-3721-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3721-7