Abstract
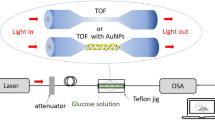
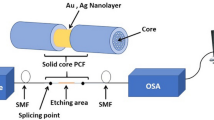
This paper proposes a novel fiber attenuated total reflection (ATR) sensor with silver nanoparticles (AgNPs) on the flattened structure based on mid-infrared spectroscopy for detecting low concentration of glucose with high precision. The flattened structure was designed to add the effective optical path length to improve the sensitivity. AgNPs were then deposited on the surface of the flattened area of the fiber via chemical silver mirror reaction for further improving the sensitivity by enhancing the infrared absorption. Combining the AgNPs modified flattened fiber ATR sensor with a CO2 laser showed a strong mid-infrared glucose absorption, with an enhancement factor of 4.30. The glucose concentration could be obtained by a five-variable partial least-squares model with a root-mean-square error of 4.42 mg/dL, which satisfies clinical requirements. Moreover, the fiber-based technique provides a pretty good method to fabricate miniaturized ATR sensors that are suitable to be integrated into a microfluidic chip for continuous glucose monitoring with high sensitivity.











Similar content being viewed by others
References
M. Ahmad, L.L. Hench, Effect of taper geometries and launch angle on evanescent wave penetration depth in optical fibers. Biosens. Bioelectron. 20(7), 1312–1319 (2005)
T. Bailey, H. Zisser, A. Chang, New features and performance of a next-generation SEVEN-day continuous glucose monitoring system with short lag time. Diabetes Technol The 11(12), 749–755 (2009)
Y.Z. Cao, W. Zhang, R. Liu, W.J. Zhang, K.X. Xu, Study of specificity for noninvasive glucose measurements based on two-dimensional correlation mid-infrared spectroscopy. Proc. SPIE 8229 (2012)
K.H. Cha, M.E. Meyerhoff, Compatibility of nitric oxide release with implantable enzymatic glucose sensors based on osmium (III/II) mediated electrochemistry. ACS Sens. 2(9), 1262–1266 (2017)
R.L.J. Chang, J. Yang, Surface-controlled Electroless deposition method in the preparation of stacked silver nanoparticles on germanium for surface-enhanced infrared absorption measurements. Appl. Spectrosc. 64(2), 211–218 (2010)
U. Damm, V.R. Kondepati, H.M. Heise, Continuous reagent-free bed-side monitoring of glucose in biofluids using infrared spectrometry and micro-dialysis. Vib. Spectrosc. 43(1), 184–192 (2007)
J.M. Delgado, J.M. Orts, J.M. Perez, A. Rodes, Sputtered thin-film gold electrodes for in situ ATR-SEIRAS and SERS studies. J. Electroanal. Chem. 617(2), 130–140 (2008)
P. Dumas, R.G. Tobin, P.L. Richards, Study of adsorption states and interactions of CO on evaporated noble metal surfaces by infrared absorption spectroscopy. II. Gold and copper. Surface Science 171(3), 579–599 (1986)
A. Hartstein, J.R. Kirtley, J.C. Tsang, Enhancement of the infrared absorption from molecular monolayers with thin metal overlayers. Phys. Rev. Lett. 45(3), 201–204 (1980)
H.M. Heise, G. Voigt, P. Lampen, L. Kupper, S. Rudloff, G. Werner, Multivariate calibration for the determination of analytes in urine using mid-infrared attenuated total reflection spectroscopy. Appl. Spectrosc. 55(4), 434–443 (2001)
D.B. Keenan, J.J. Mastrototaro, S.A. Weinzimer, G.M. Steil, Interstitial fluid glucose time-lag correction for real-time continuous glucose monitoring. Biomed Signal Proces 8(1), 81–89 (2013)
N. Khedmi, M. Ben Rabeh, M. Kanzari, Structural morphological and optical properties of SnSb2S4 thin films grown by vacuum evaporation method. J. Mater. Sci. Technol. 30(10), 1006–1011 (2014)
H. von Lilienfeld-Toal, M. Weidenmuller, A. Xhelaj, W. Mantele, A novel approach to non-invasive glucose measurement by mid-infrared spectroscopy: The combination of quantum cascade lasers (QCL) and photoacoustic detection. Vib. Spectrosc. 38(1–2), 209–215 (2005)
A.I. Lopez-Lorente, M. Sieger, M. Valcarcel, B. Mizaikoff, Infrared attenuated total reflection spectroscopy for the characterization of gold nanoparticles in solution. Anal. Chem. 86(1), 783–789 (2014)
C. Lu, L. Xingbo, L. Yang, Study of influencing factors on vacuum evaporation film thickness. Appl. Mech. Mater. 536-537, 3716–3720 (2014)
J. Mastrototaro, J. Shin, A. Marcus, G. Sulur, S.C.T. Investigator, The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol The 10(5), 385–390 (2008)
G.T. Merklin, P.R. Griffiths, Influence of chemical interactions on the surface-enhanced infrared absorption spectrometry of nitrophenols on copper and silver films. Langmuir 13(23), 6159–6163 (1997)
M. Osawa, Dynamic processes in electrochemical reactions studied by surface-@enhanced infrared absorption spectroscopy (SEIRAS). Bull. Chem. Soc. Jpn. 70(12), 2861–2861 (1997)
M. Osawa, in Near-Field Optics and Surface Plasmon Polaritons, ed. by S. Kawata. Surface-enhanced infrared absorption (Springer Berlin Heidelberg, Berlin, Heidelberg, 2001), pp. 163–187
M. Pleitez, H. von Lilienfeld-Toal, W. Mantele, Infrared spectroscopic analysis of human interstitial fluid in vitro and in vivo using FT-IR spectroscopy and pulsed quantum cascade lasers (QCL): Establishing a new approach to non invasive glucose measurement. Spectrochim. Acta A 85(1), 61–65 (2012)
Y. Raichlin, L. Fel, A. Katzir, Evanescent-wave infrared spectroscopy with flattened fibers as sensing elements. Opt. Lett. 28(23), 2297–2299 (2003)
Y. Raichlin, D. Avisar, L. Gerber, A. Katzir, Flattened infrared fiber-optic sensors for the analysis of micrograms of insoluble solid particles in solution or in a dry state. Vib. Spectrosc. 73, 67–72 (2014)
G.P.C. Rao, J. Yang, Preparation of high-capacity substrates from polycrystalline silver chloride for the selective detection of tyrosine by surface-enhanced infrared absorption (SEIRA) measurements. Anal. Bioanal. Chem. 401(9), 2935–2943 (2011)
K. Rebrin, G.M. Steil, Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol The 2(3), 461–472 (2000)
R. Rosipal, N. Kramer, Overview and recent advances in partial least squares. Lect Notes Comput Sc 3940, 34–51 (2006)
T. Shi, D. Li, G. Li, Y. Zhang, K. Xu, L. Lu, Modeling and measurement of correlation between blood and interstitial glucose changes. Journal of diabetes research 2016, 1 (2016)
F. Verger, T. Pain, V. Nazabal, C. Boussard-Pledel, B. Bureau, F. Colas, E. Rinnert, K. Boukerma, C. Compere, M. Guilloux-Viry, S. Deputier, A. Perrin, J.P. Guin, Surface enhanced infrared absorption (SEIRA) spectroscopy using gold nanoparticles on As2S3 glass. Sensor Actuat B-Chem 175, 142–148 (2012)
D.M. Wilson, R.W. Beck, W.V. Tamborlane, M.J. Dontchev, C. Kollman, P. Chase, L.A. Fox, K.J. Ruedy, E. Tsalikian, S.A. Weinzimer, The accuracy of the FreeStyle navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care 30(1), 59–64 (2007)
H.X. Yu, D.C. Li, R.C. Roberts, K.X. Xu, N.C. Tien, An interstitial fluid transdermal extraction system for continuous glucose monitoring. J Microelectromech S 21(4), 917–925 (2012)
S.L. Yu, D.C. Li, H. Chong, C.Y. Sun, K.X. Xu, Continuous glucose determination using fiber-based tunable mid-infrared laser spectroscopy. Opt Laser Eng 55, 78–83 (2014a)
S.L. Yu, D.C. Li, H. Chong, C.Y. Sun, H.X. Yu, K.X. Xu, In vitro glucose measurement using tunable mid-infrared laser spectroscopy combined with fiber-optic sensor. Biomed Opt Express 5(1), 275–286 (2014b)
Funding
This work was supported by the National Natural Science Foundation of China (No.81571766), the National Key Research and Development Program of China (No.2017YFA0205103), and the 111 Project of China (No.B07014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest related to this article.
Rights and permissions
About this article
Cite this article
Li, W., Sun, C., Yu, S. et al. Flattened fiber-optic ATR sensor enhanced by silver nanoparticles for glucose measurement. Biomed Microdevices 20, 104 (2018). https://doi.org/10.1007/s10544-018-0346-9
Published:
DOI: https://doi.org/10.1007/s10544-018-0346-9