Abstract
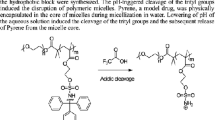
A novel synthetic strategy towards pH-responsive aggregation-induced emission (AIE)-active tetraphenylethene (TPE)-functionalized polyethylene-based block copolymers is presented. Tris(3-(4-(1,2,2-triphenylvinyl)phenoxy)propyl)borane was used to initiate the polyhomologation of dimethylsulfoxonium methylide to afford well-defined α-TPE-ω-OH linear polyethylenes (PE). The terminal hydroxyl groups were transformed to atom transfer radical polymerization (ATRP) initiating sites by esterification with α-bromoisobutyryl bromide, followed by polymerization of tert-butyl acrylate (tBA) to provide TPE-PE-b-PtBA block copolymers. After hydrolysis of the tBu group to COOH group, the corresponding pH-responsive TPE-PE-b-PAA block copolymers were obtained. All synthesized block copolymers revealed AIE behavior either in solution or bulk. Due to the pH-responsivity of PAA chains, the aggregation state at different pH and consequently the fluorescence intensity changed. Also, the synthesized block copolymers exhibited ion-specificity fluorescence properties.
Similar content being viewed by others
References
Bünzli, J. C. G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755.
Jüstel, T.; Nikol, H.; Ronda, C. New developments in the field of luminescent materials for lighting and displays. Angew. Chem. Int. Ed. 1998, 37, 3084–3103.
Schmidt, A.; Anderson, M.; Armstrong, N. R. Electronic states of vapor deposited electron and hole transport agents and luminescent materials for light-emitting diodes. J. Appl. Phys. 1995, 78, 5619–5625.
Bredol, M.; Kynast, U.; Ronda, C. Designing luminescent materials. Adv. Mater. 1991, 3, 361–367.
Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740, 1740–1741.
Förster, T.; Kasper, K. Ein konzentrationsumschlag der fluoreszenz des pyrens. Zeitschrift für Elektrochemie, Berichte der Bunsengesellschaft für physikalische Chemie 1955, 59, 976–980.
Feng, H. T.; Yuan, Y. X.; Xiong, J. B.; Zheng, Y. S.; Tang, B. Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem. Soc. Rev. 2018, 47, 7452–7476.
Mei, J.; Leung, N. L.; Kwok, R. T.; Lam, J. W. Y.; Tang, B. Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940.
Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388.
Qin, A.; Lam, J. W. Y.; Tang, B. Z. Luminogenic polymers with aggregation-induced emission characteristics. Prog. Polym. Sci. 2012, 37, 182–209.
Hu, R.; Leung, N. L.; Tang, B. Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562.
Stuart, M. A. C.; Huck, W. T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G. B.; Szleifer, I.; Tsukruk, V. V.; Urban, M. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113.
Liu, F.; Urban, M. W. Recent advances and challenges in designing stimuli-responsive polymers. Prog. Polym. Sci. 2010, 35, 3–23.
Lendlein, A.; Shastri, V. P. Stimuli-sensitive polymers. Adv. Mater. 2010, 22, 3344–3347.
De las Heras Alarcón, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285.
Roth, P. J.; Lowe, A. B. Stimulus-responsive polymers. Polym. Chem. 2017, 8, 10–11.
Kocak, G.; Tuncer, C.; Bütün, V. pH-responsive polymers. Polym. Chem. 2017, 8, 144–176.
Wei, M.; Gao, Y.; Li, X.; Serpe, M. J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143.
McLaurin, E. J.; Bradshaw, L. R.; Gamelin, D. R. Dual-emitting nanoscale temperature sensors. Chem. Mater. 2013, 25, 1283–1292.
Cui, Y.; Song, R.; Yu, J.; Liu, M.; Wang, Z.; Wu, C.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Dual-emitting MOF dye composite for ratiometric temperature sensing. Adv. Mater. 2015, 27, 1420–1425.
Zhang, Z.; Hadjichristidis, N. Temperature and pH-dual responsive AIE-active core crosslinked polyethylene-poly (methacrylic acid) multimiktoarm star copolymers. ACS Macro Lett. 2018, 7, 886–891.
Shea, K. J. Polyhomologation: The living polymerization of ylides. Chem. Eur. J. 2000, 6, 1113–1119.
Shea, K.; Walker, J.; Zhu, H.; Paz, M.; Greaves, J. Polyhomologation A living polymethylene synthesis. J. Am. Chem. Soc. 1997, 119, 9049–9050.
Luo, J.; Shea, K. J. Polyhomologation. A living C1 polymerization. Acc. Chem. Res. 2010, 43, 1420–1433.
Wang, D.; Hadjichristidis, N. Terpolymers from borane-initiated copolymerization of triphenyl arsonium and sulfoxonium ylides: An unexpected light emission. Angew. Chem. 2019, 131, 6361–6365.
Jiang, Y.; Zhang, Z.; Wang, D.; Hadjichristidis, N. An efficient and general strategy toward the synthesis of polyethylene-based cyclic polymers. Macromolecules 2018, 51, 3193–3202.
Xue, Y.; Lu, H. C.; Zhao, Q. L.; Huang, J.; Xu, S. G.; Cao, S. K.; Ma, Z. Polymtmylene-b-poly(ttyeene-co-2,3,4,5,6-pentafluoro styrene) copolymers: Synthesis and fabrication of their porous films. Polym. Chem. 2013, 4, 307–312.
He, Q.; Ren, J.; Ren, J.; Pang, K.; Ma, Z.; Zhu, X.; Song, R. Polymethylene-b-poly(acrylic acid) diblock copolymers: Aggregation and crystallization in the presence of CaCl2. Eur. Polym. J. 2017, 95, 174–185.
Wang, H.; Xu, F.; Cui, K.; Zhang, H.; Huang, J.; Zhao, Q.; Jiang, T.; Ma, Z. Synthesis of polymethylene-b-poly(vinyl acetate) block copolymer via visible light induced radical polymerization and its application. RSC Adv. 2017, 7, 42484–42490.
Xue, Y.; Zhang, S. S.; Cui, K.; Huang, J.; Zhao, Q. L.; Lan, P.; Cao, S. K.; Ma, Z. New polymethylene-based AB2 star copolymers synthesized via a combination of polyhomologation of ylides and atom transfer radical polymerization. RSC Adv. 2015, 5, 7090–7097.
Wang, D.; Hadjichristidis, N. Allyl borates: A novel class of polyhomologation initiators. Chem. Commun. 2017, 53, 1196–1199.
Zhang, H.; Banerjee, S.; Faust, R.; Hadjichristidis, N. Living cationic polymerization and polyhomologation: An ideal combination to synthesize functionalized polyethylene-polyisobutylene block copolymers. Polym. Chem. 2016, 7, 1217–1220.
Zhang, Z.; Altaher, M.; Zhang, H.; Wang, D.; Hadjichristidis, N. Synthesis of well-defined polyethylene-based 3-miktoarm star copolymers and terpolymers. Macromolecules 2011, 49, 2630–2638.
Zhang, Z.; Gnanou, Y.; Hadjichristidis, N. Well-defined 4-arm stars with hydroxy-terminated polyethylene, polyethylene-b-polycaprolactone and polyethylene-b-(polymethyl methacrylate) 2 arms. Polym. Chem. 2011, 7, 5507–5511.
Zhang, Z.; Zhang, H.; Gnanou, Y.; Hadjichristidis, N. Polyhomologation based on in situ generated boron-thexyl-silaboracyclic initiating sites: A novel strategy towards the synthesis of polyethylene-based complex architectures. Chem. Commun. 2015, 51, 9936–9938.
Zhang, H.; Gnanou, Y.; Hadjichristidis, N. Well-defined polyethylene molecular brushes by polyhomologation and ring opening metathesis polymerization. Polym. Chem. 2014, 5, 6431–6434.
Wang, D.; Zhang, Z.; Hadjichristidis, N. C1 polymerization: A unique tool towards polyethylene-based complex macromolecular architectures. Polym. Chem. 2017, 8, 4062–4073.
Jiang, Y.; Hadjichristidis, N. Tetraphenylethene-functionalized polyethylene-based polymers with aggregation-induced emission. Macromolecules 2019, 52, 1955–1964.
Corey, E.; Chaykovsky, M. Dimethyloxosulfonium methylide ((CH3)2SOCH2) and dimethylsulfonium methylide ((CH3)2SCH2). Formation and application to organic synthesis. J. Am. Chem. Soc. 1965, 87, 1353–1364.
Zhang, H.; Alkayal, N.; Gnanou, Y.; Hadjichristidis, N. Anionic polymerization and polyhomologation: An ideal combination to synthesize polyethylene-based block copolymers. Chem. Commun. 2013, 49, 8952–8954.
Guan, X.; Zhang, D.; Meng, L.; Zhang, Y.; Jia, T.; Jin, Q.; Wei, Q.; Lu, D.; Ma, H. Various tetraphenylethene-based aiegens with four functional polymer arms: Versatile synthetic approach and photophysical properties. Ind. Eng. Chem. Res. 2017, 56, 680–686.
Chen, F.; Li, C.; Wang, X.; Liu, G.; Zhang, G. pH and ion-species sensitive fluorescence properties of star polyelectrolytes containing a triphenylene core. Soft Matter 2012, 8, 6364–6370.
Acknowledgments
The research reported in this publication was supported by King Abdullah University of Science and Technology (KAUST).
Author information
Authors and Affiliations
Corresponding author
Additional information
Invited article for special issue of “Ionic Polymerization”
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jiang, Y., Hadjichristidis, N. pH-responsive AIE-active Polyethylene-based Block Copolymers. Chin J Polym Sci 37, 930–935 (2019). https://doi.org/10.1007/s10118-019-2330-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-019-2330-0