Abstract
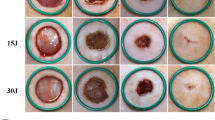
The aim of the study was to explore the effect and mechanism of a low-level laser on hair follicle stem cells in full-thickness skin wound healing in mice. Full-thickness skin defects were generated by a 5-mm punch biopsy tool on the backs of depilated C57/BL6N mice, which were randomly divided thereafter into a low-dose laser treatment group (LLLT-Low), a high-dose laser treatment group (LLLT-High), and a control group (control). From the day of modeling to the day before the skin samples were taken, the wound area and wound edge of the mice in the LLLT-Low and LLLT-High groups were irradiated with a laser comb every 24 h, and the energy density was 1 J/cm2 and 10 J/cm2, respectively. The control group was irradiated with an ordinary fluorescent lamp. At 0, 3, 5, 10, and 14 days after modeling, pictures of each wound were taken, and the percent wound closure was analyzed. At 3, 5, 10, and 14 days after modeling, the samples were observed by hematoxylin and eosin (HE) and immunofluorescence (IF) staining. Whole transcriptome sequencing (RNA-Seq) was performed on the samples on day 10. Gene Ontology (GO) analysis was performed, and the results were validated by Western blot analysis and enzyme-linked immunosorbent assay (ELISA). The analysis of the percent of wound closure showed that healing was accelerated (significantly from 5 to 10 days) in the LLLT-Low group, but there was no clear change in the LLLT-High group. HE staining showed that the LLLT-Low group had an increasing number of hair follicles and a tendency to migrate to the center of the wound. There was no significant increase in the number of hair follicles and no obvious migration in the LLLT-High group. Immunofluorescence staining showed that the total number of CK15 + hair follicle stem cells in the LLLT-Low group was higher than that in the control group and LLLT-High group at all time points. The number and farthest migration distance of CK15 + hair follicle stem cells increased significantly with time, and after 5 days, they were significantly higher than those in the control group and LLLT-High group. RNA-Seq and Western blot analysis showed that the expression of related genes in hair follicle stem cells, including CK15, in the LLLT-Low group was upregulated. GO analysis and ELISA showed that the expression of many cytokines, represented by IL34, in the LLLT-Low group was upregulated. Low-level laser treatment can promote the proliferation, differentiation, and migration of CK15 + hair follicle stem cells by upregulating the cytokine IL34, thereby promoting skin wound healing in mice.





Similar content being viewed by others
References
Boyko TV, Longaker MT, Yang GP (2017) Laboratory models for the study of normal and pathologic wound healing. Plast Reconstr Surg 139:654–662. https://doi.org/10.1097/PRS.0000000000003077
Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S et al (2009) Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4:427–439. https://doi.org/10.1016/j.stem.2009.04.014
Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascre G, Simons BD et al (2017) Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun 8:14684. https://doi.org/10.1038/ncomms14684
Donati G, Rognoni E, Hiratsuka T, Liakath-Ali K, Hoste E, Kar G et al (2017) Wounding induces dedifferentiation of epidermal Gata6(+) cells and acquisition of stem cell properties. Nat Cell Biol 19:603–613. https://doi.org/10.1038/ncb3532
Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N et al (2012) Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150:136–150. https://doi.org/10.1016/j.cell.2012.04.045
Dompe C, Moncrieff L, Matys J, Grzech-Lesniak K, Kocherova I, Bryja A et al (2020) Photobiomodulation-underlying mechanism and clinical applications. J Clin Med 9. https://doi.org/10.3390/jcm9061724
Zhang T, Liu L, Fan J, Tian J, Gan C, Yang Z et al (2017) Low-level laser treatment stimulates hair growth via upregulating Wnt10b and beta-catenin expression in C3H/HeJ mice. Lasers Med Sci 32:1189–1195. https://doi.org/10.1007/s10103-017-2224-8
Torres AE, Lim HW (2021) Photobiomodulation for the management of hair loss. Photodermatol Photoimmunol Photomed 37:91–98. https://doi.org/10.1111/phpp.12649
Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR (2014) Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med 46:144–151. https://doi.org/10.1002/lsm.22170
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321. https://doi.org/10.1038/nature07039
Figurova M, Ledecky V, Karasova M, Hluchy M, Trbolova A, Capik I et al (2016) Histological assessment of a combined low-level laser/light-emitting diode therapy (685 nm/470 nm) for sutured skin incisions in a porcine model: a short report. Photomed Laser Surg 34:53–55. https://doi.org/10.1089/pho.2015.4013
Li Y, Zhang J, Xu Y, Han Y, Jiang B, Huang L et al (2016) The histopathological investigation of red and blue light emitting diode on treating skin wounds in Japanese big-ear white rabbit. PLoS ONE 11:e0157898. https://doi.org/10.1371/journal.pone.0157898
Tripodi N, Corcoran D, Antonello P, Balic N, Caddy D, Knight A et al (2021) The effects of photobiomodulation on human dermal fibroblasts in vitro: a systematic review. J Photochem Photobiol B 214:112100. https://doi.org/10.1016/j.jphotobiol.2020.112100
Guo Y, Qu Q, Chen J, Miao Y, Hu Z (2020) Proposed mechanisms of low-level light therapy in the treatment of androgenetic alopecia. Lasers Med Sci. https://doi.org/10.1007/s10103-020-03159-z
Hwang I, Choi KA, Park HS, Jeong H, Kim JO, Seol KC et al (2016) Neural stem cells restore hair growth through activation of the hair follicle niche. Cell Transplant 25:1439–1451. https://doi.org/10.3727/096368916X691466
Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL (2011) Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8:552–565. https://doi.org/10.1016/j.stem.2011.02.021
Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ et al (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11:1351–1354. https://doi.org/10.1038/nm1328
Rompolas P, Mesa KR, Greco V (2013) Spatial organization within a niche as a determinant of stem-cell fate. Nature 502:513–518. https://doi.org/10.1038/nature12602
Hoeck JD, Biehs B, Kurtova AV, Kljavin NM, De Sousa EMF, Alicke B et al (2017) Stem cell plasticity enables hair regeneration following Lgr5(+) cell loss. Nat Cell Biol 19:666–676. https://doi.org/10.1038/ncb3535
Langton AK, Herrick SE, Headon DJ (2008) An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol 128:1311–1318. https://doi.org/10.1038/sj.jid.5701178
Nowak JA, Polak L, Pasolli HA, Fuchs E (2008) Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3:33–43. https://doi.org/10.1016/j.stem.2008.05.009
Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y et al (2011) The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144:577–589. https://doi.org/10.1016/j.cell.2011.01.014
Wu S, Zhou F, Wei Y, Chen WR, Chen Q, Xing D (2014) Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal 20:733–746. https://doi.org/10.1089/ars.2013.5229
Yang WZ, Chen JY, Yu JT, Zhou LW (2007) Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem Photobiol 83:979–984. https://doi.org/10.1111/j.1751-1097.2007.00116.x
Albert ES, Bec JM, Desmadryl G, Chekroud K, Travo C, Gaboyard S et al (2012) TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J Neurophysiol 107:3227–3234. https://doi.org/10.1152/jn.00424.2011
De Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22. https://doi.org/10.1109/JSTQE.2016.2561201
Liao X, Xie GH, Liu HW, Cheng B, Li SH, Xie S et al (2014) Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: proposed mechanism for enhanced wound re-epithelialization. Photomed Laser Surg 32:219–225. https://doi.org/10.1089/pho.2013.3667
Fernandes AP, JunqueiraMde A, Marques NC, Machado MA, Santos CF, Oliveira TM et al (2016) Effects of low-level laser therapy on stem cells from human exfoliated deciduous teeth. J Appl Oral Sci 24:332–337. https://doi.org/10.1590/1678-775720150275
Huang YY, Chen AC, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose Response 7:358–383. https://doi.org/10.2203/dose-response.09-027.Hamblin
Huang YY, Sharma SK, Carroll J, Hamblin MR (2011) Biphasic dose response in low level light therapy - an update. Dose Response 9:602–618. https://doi.org/10.2203/dose-response.11-009.Hamblin
Baghdadi M, Umeyama Y, Hama N, Kobayashi T, Han N, Wada H et al (2018) Interleukin-34, a comprehensive review. J Leukoc Biol 104:931–951. https://doi.org/10.1002/JLB.MR1117-457R
Guillonneau C, Bezie S, Anegon I (2017) Immunoregulatory properties of the cytokine IL-34. Cell Mol Life Sci 74:2569–2586. https://doi.org/10.1007/s00018-017-2482-4
Baghdadi M, Endo H, Tanaka Y, Wada H, Seino KI (2017) Interleukin 34, from pathogenesis to clinical applications. Cytokine 99:139–147. https://doi.org/10.1016/j.cyto.2017.08.020
Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M et al (2012) IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13:753–760. https://doi.org/10.1038/ni.2360
Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY et al (2012) Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 13:744–752. https://doi.org/10.1038/ni.2353
Masson-Meyers DS, TaM A, Caetano GF, Guimaraes FR, Leite MN, Leite SN et al (2020) Experimental models and methods for cutaneous wound healing assessment. Int J Exp Pathol 101:21–37. https://doi.org/10.1111/iep.12346
Davidson JM, Yu F, Opalenik SR (2013) Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care (New Rochelle) 2:142–148. https://doi.org/10.1089/wound.2012.0424
Wang X, Ge J, Tredget EE, Wu Y (2013) The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc 8:302–309. https://doi.org/10.1038/nprot.2013.002
Agha R, Ogawa R, Pietramaggiori G, Orgill DP (2011) A review of the role of mechanical forces in cutaneous wound healing. J Surg Res 171:700–708. https://doi.org/10.1016/j.jss.2011.07.007
Chen L, Mirza R, Kwon Y, Dipietro LA, Koh TJ (2015) The murine excisional wound model: contraction revisited. Wound Repair Regen 23:874–877. https://doi.org/10.1111/wrr.12338
Zomer HD, Trentin AG (2018) Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 90:3–12. https://doi.org/10.1016/j.jdermsci.2017.12.009
Chu GY, Chen YF, Chen HY, Chan MH, Gau CS, Weng SM (2018) Stem cell therapy on skin: mechanisms, recent advances and drug reviewing issues. J Food Drug Anal 26:14–20. https://doi.org/10.1016/j.jfda.2017.10.004
Kim KH, Pierce MC, Maguluri G, Park BH, Yoon SJ, Lydon M et al (2012) In vivo imaging of human burn injuries with polarization-sensitive optical coherence tomography. J Biomed Opt 17:066012. https://doi.org/10.1117/1.JBO.17.6.066012
Deegan AJ, Wang W, Men S, Li Y, Song S, Xu J et al (2018) Optical coherence tomography angiography monitors human cutaneous wound healing over time. Quant Imaging Med Surg 8: 135–150. https://doi.org/10.21037/qims.2018.02.07
Lu J, Deegan AJ, Cheng Y, Liu T, Zheng Y, Mandell SP et al (2021) Application of OCT-derived attenuation coefficient in acute burn-damaged skin. Lasers Surg Med. https://doi.org/10.1002/lsm.23415
Fan Y, Ma Q, Xin S, Peng R, Kang H (2021) Quantitative and qualitative evaluation of supercontinuum laser-induced cutaneous thermal injuries and their repair with OCT images. Lasers Surg Med 53:252–262. https://doi.org/10.1002/lsm.23287
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019) Wound healing: a cellular perspective. Physiol Rev 99:665–706. https://doi.org/10.1152/physrev.00067.2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study involving animals were approved by the Animal Care and Use Committee of the Chinese Academy of Medical Sciences (No. 2020200).
Conflict of interest
The authors declare no competing interests.
Role of funding resources
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Liu, L., Fan, J. et al. Low-level laser treatment promotes skin wound healing by activating hair follicle stem cells in female mice. Lasers Med Sci 37, 1699–1707 (2022). https://doi.org/10.1007/s10103-021-03419-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03419-6