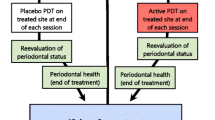
Abstract
This study investigated the local effect of photobiomodulation (PBM) for the treatment of periodontal pockets in patients with periodontitis and type 2 diabetes. Thirty-eight periodontal pockets presenting probing depth (PD) and clinical attachment level (CAL) ≥ 5 mm were selected from 19 patients (two pockets/patient). The selected periodontal pockets were randomly assigned to receive mechanical debridement only (control group) or mechanical debridement with PBM (PBM group). Clinical measures, such as PD, CAL, bleeding on probing (BoP), and presence of supragingival biofilm (PI), were collected and compared at baseline, 3, 6, and 12 months. After 12 months, no statistically difference was observed for mean PD and mean CAL when control and PBM groups were compared. The frequency of pockets with PD 5–6 mm was significantly lower for the PBM group at 6 months when compared to the control group. Pockets with PD ≥ 7 mm changed significantly between baseline and 3, 6, and 12 months for the PBM group, while for the control group, statistical significance was only observed between baseline and 6 months. The PBM protocol used in this study did not provide significant changes for PD and CAL in periodontal pockets when compared to mechanical therapy only. However, PBM was more effective in reducing the percentage of moderate periodontal pockets at 6 months in patients with type 2 DM.


Similar content being viewed by others
References
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6
Cobb CM (1996) Non-surgical pocket therapy: mechanical. Ann Periodontol 1:443–490. https://doi.org/10.1902/annals.1996.1.1.443
Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS (1997) Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Peiodontol 2000 14:216–248
Chapple ILC, Genco R, on behalf of the working group 2 of the joint EFP/AAP workshop (2013) Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol 84:S106–S112. https://doi.org/10.1902/jop.2013.1340011
Lalla E, Lamster IB, Stern DM, Schmidt AM (2001) Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetes: mechanisms and insights into therapeutics modalities. Ann Periodontol 6(1):113–118
Nassar H, Kantarci A, Van Dyke TE (2007) Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000 43:233–244
Duarte PM, Bezerra JP, Miranda TS, Feres M, Chambrone L, Shaddox LM (2014) Local levels of inflammatory mediators in uncontrolled type 2 diabetic subjects with chronic periodontitis. J Clin Periodontol 41(1):11–18. https://doi.org/10.1111/jcpe.12179
Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes AB Jr, Taba M Jr (2010) Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. J Periodontol 81(3):384–391. https://doi.org/10.1902/jop.2009.090510
Bastos MF, Tucci MA, de Siqueira A, de Faveri M, Figueiredo LC, Vallim PC, Duarte PM (2016) Diabetes may affect the expression of matrix metalloproteinase and their inhibitors more than smoking in chronic periodontitis. J Periodontal Res. https://doi.org/10.1111/jre.12394
Westfelt E, Rylander H, Blohmé G, Jonasson P, Lindhe J (1996) The effect of periodontal therapy in diabetics. Results after 5 years. J Clin Periodontol 23:92–100
Kiran M, Arpak N, Unsal E, Erdogan MF (2005) The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol 32:266–272. https://doi.org/10.1111/j.1600-051X.2005.00658.x
Singh S, Kumar V, Kumar S, Subbappa A (2008) The effect of periodontal therapy on the improvement of glycaemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes Dev Ctries 28:38–44. https://doi.org/10.4103/0973-3930.43097
Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN (2011) A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. J Clin Periodontol 38:142–147. https://doi.org/10.1111/j.1600-051X.2010.01652.x
Santos VR, Lima JA, Miranda TS, Gonçalves TE, Figueiredo LC, Faveri M, Duarte PM (2013) Full-mouth disinfections a therapeutic protocol for type-2 diabetic subjects with chronic periodontitis: twelve-month outcomes. A randomized clinical trial. J Clin Periodontol 40(2):155–162. https://doi.org/10.1111/jcpe.12040
Castro dos Santos NC, Andere NM, Araujo CF, de Marco AC, Dos Santos LM, Jardini MA, Santamaria MP (2016) Local adjunct effect of antimicrobial photodynamic therapy for the treatment of chronic periodontitis in type 2 diabetics: split-mouth double-blind randomized controlled clinical trial. Lasers Med Sci 31(8):1633–1640. https://doi.org/10.1007/s10103-016-2030-8
Tamashiro NS, Duarte PM, Miranda TS, Maciel SS, Figueiredo LC, Faveri M, Feres M. Amoxicillin plus metronidazole therapy for patients with periodontitis and type 2 diabetes: a 2-year randomized controlled trial. J Dent Res 2016;95(7):829–836. doi: 10.1177/0022034516639274
Dias SB, Fonseca MV, Dos Santos NC, Mathias IF, Martinho FC, Junior MS, Jardini MA, Santamaria MP (2015) Effect of GaAIAs low-level laser therapy on the healing of human palate mucosa after connective tissue graft harvesting: randomized clinical trial. Lasers Med Sci 30(6):1695–1702. https://doi.org/10.1007/s10103-014-1685-2
da Silva Neves FL, Silveira CA, Dias SB, Santamaria Júnior M, de Marco AC, Kerbauy WD, de Melo Filho AB, Jardini MA, Santamaria MP (2016) Comparison of two power densities on the healing of palatal wounds after connective tissue graft removal: randomized clinical trial. Lasers Med Sci 31(7):1371–1378. https://doi.org/10.1007/s10103-016-1988-6
Fernandes-Dias SB, de Marco AC, Santamaria M Jr, Kerbauy WD, Jardini MA, Santamaria MP (2015) Connective tissue graft associated or not with low laser therapy to treat gingival recession: randomized clinical trial. J Clin Periodontol 42(1):54–61. https://doi.org/10.1111/jcpe.12328
Santamaria MP, Fernandes-Dias SB, Araújo CF, da Silva Neves FL, Mathias IF, Rebelato Bechara Andere NM, Neves Jardini MA (2017) 2-year assessment of tissue bioestimulation with low-level laser on the outcomes of connective tissue graft in the treatment of single gingival recession. Randomized clinical trial. J Periodontol 88(4):320-328. https://doi.org/10.1902/jop.2016.160391
Carrillo JS, Calatayud J, Manso FJ, Barberia E, Martinez JM, Donado M (1990) A randomized double-blind clinical trial on the effectiveness of helium-neon laser in the prevention of pain, swelling and trismus after removal of impacted third molars. Int Dent J 40(1):31–36
Hennessy M, Hamblin MR (2017) Photobiomodulation in the brain: a new paradigm. J Opt 19(1):013003. https://doi.org/10.1088/2040-8986/19/1/013003
Wang X, Tian F, Soni SS, Gonzalez-Lime F, Liu H Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 3(6):30540. https://doi.org/10.1038/srep30540
de Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3). https://doi.org/10.1109/JSTQE.2016.2561201
Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22(5):302–311
Kreisler M, Al Haj H, d'Hoedt B (2005) Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med 37(5):350–355
Dukić W, Bago I, Aurer A, Roguljić M (2013) Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. J Periodontol 84(8):1111–1117. https://doi.org/10.1902/jop.2012.110708
Makhlouf M, Dahaba MM, Tunér J, Eissa SA, Harhash TA (2012) Effect of adjunctive low-level laser therapy (LLLT) on nonsurgical treatment of chronic periodontitis. Photomed Laser Surg 30(3):160–166. https://doi.org/10.1089/pho.2011.3069
Saglam M, Kantarci A, Dundar N, Hakki SS (2014) Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29(1):37–46. https://doi.org/10.1007/s10103-012-1230-0
Koçak E, Sağlam M, Kayış SA, Dündar N, Kebapçılar L, Loos BG, Hakkı SS (2016) Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci 31(2):343–353. https://doi.org/10.1007/s10103-016-1868-0
Fekrazad R, Mirmoezzi A, Kalhori KA, Arany P (2015) The effect of red, green and blue lasers on healing of oral wounds in diabetic rats. J Photochem Photobiol B 148:242–245. https://doi.org/10.1016/j.jphotobiol.2015.04.018
Fahimipour F, Houshmand B, Alemi P, Asnaashari M, Tafti MA, Akhoundikharanagh F, Farashah SE, Aminisharifabad M, Korani AS, Mahdian M, Bastami F, Tahriri M (2016) The effect of He-Ne and Ga-Al-As lasers on the healing of oral mucosa in diabetic mice. J Photochem Photobiol B 159:149–154. https://doi.org/10.1016/j.jphotobiol.2016.03.020
Julius SA (2004) Sample sizes for clinical trials with Normal data. Stat Med 23:1921–1986
Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ et al (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 63:e1–e37. https://doi.org/10.1016/j.jclinepi.2010.03.004
Koçak E, Sağlam M, Kayış SA, Dündar N, Kebapçılar L, Loos BG, Hakkı SS (2016) Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics in chronic periodontitis: a randomized clinical trial. Lasers Med Sci 31(2):343–353. https://doi.org/10.1007/s10103-016-1868-0
Schwarz F, Aoki A, Sculean A, Becker J (2009) The impact of laser application on periodontal and peri-implant wound healing. Periodontol 2000 51:79–108. https://doi.org/10.1111/j.1600-0757.2009.00301.x
Cobb MC (2017) Lasers and the treatment of periodontitis: the essence and the noise. Periodontol 2000 75(1):205–295. https://doi.org/10.1111/pdr.12137
Cheng Y, Chen JW, Ge MK, Zhou ZY, Yin X, Zou SJ (2016) Efficacy of adjunctive laser in non-surgical periodontal treatment: a systematic review and meta-analysis. Lasers Med Sci 31(1):151–163. https://doi.org/10.1007/s10103-015-1795-5
Abduljabbar T, Javed F, Shah A, Samer MS, Vohra F, Akram A (2017) Role of lasers as an adjunct to scaling and root planing in patients with type 2 diabetes mellitus: a systematic review. Lasers Med Sci 32(2):449–459. https://doi.org/10.1007/s10103-016-2086-5
Maiya GA, Kumar P, Rao L (2005) Effect of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamcs. Photomed Laser Surg 23(2):187–190
Reddy GK, Stehno-Bittel L, Enwemeka CS (2001) Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen 9(3):248–255
Obradović R, Kesić L, Mihailović D, Jovanović G, Antić S, Brkić Z (2012) Low-level lasers as an adjunct in periodontal therapy in patients with diabetes mellitus. Diabetes Technol Ther 14(9):799–803. https://doi.org/10.1089/dia.2012.0027
Wang X, Reddy DD, Nalawade SS, Pal S, Gonzalez-Lima F, Liu H (2018) Impact of heat on metabolic and hemodynamic changes in transcranial infrared laser stimulation measured by broadband near-infrared spectroscopy. Neurophotonics. 5(1):011004. https://doi.org/10.1117/1.NPh.5.1.011004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest. This study did not receive any funding.
Declarations of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Castro dos Santos, N., Andere, N.M.R.B., Miguel, M.M.V. et al. Photobiomodulation for the treatment of periodontal pockets in patients with type 2 diabetes: 1-year results of a randomized clinical trial. Lasers Med Sci 34, 1897–1904 (2019). https://doi.org/10.1007/s10103-019-02799-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02799-0