Abstract
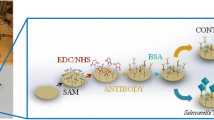
The performance of biosensors depends directly on the strategies adopted during their development. In this paper, a fast and sensitive biosensor for Salmonella Typhimurium detection was assembled by using optimization studies in separate stages. The pre-treatment assays, biomolecular immobilization (primary antibody and protein A concentrations), and analytical response (hydroquinone and hydrogen peroxide concentrations) were optimized via voltammetric methods. In the biosensor assembly, a gold surface was modified via the self-assembled monolayer technique (SAM) using cysteamine thiol and protein A for immobilization of anti-Salmonella antibody. The analytical response of the biosensor was obtained through the use of a secondary antibody labeled with a peroxidase enzyme, and the signal was evaluated by applying the chronoamperometry technique. The biosensor was characterized by infrared spectroscopy and cyclic voltammetry. Optimization of protein A and primary antibody concentrations enabled higher analytical signals of 7.5 and 75 mg mL−1, respectively, to be achieved. The hydroquinone and H2O2 concentrations selected were 3 and 300 mM, respectively. The biosensor developed attained a very low detection limit of 10 CFU mL−1 and a fast response with a final detection time of 125 min. These results indicate that this biosensor is very promising for the food safety and emergency response applications.








Similar content being viewed by others
References
Centers for disease control and prevention - CDC (2012) Pathogens causing US foodborne illnesses, hospitalizations, and deaths, 2000–2008 https://www.cdc.gov/foodborneburden/PDFs/pathogens-complete-list-01-12.pdf. Accessed 10 Apr 2017
Andrews WH, Wang H, Jacobson A, Hammack TS (2016) Salmonella. In: FOOD AND DRUG ADMINISTRATION. Bacteriological analytical manual (BAM) on line. Chap. 5. https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149.htm>. Accessed 12 Apr 2017
Velusamy V, Arshak K, Korostynska O, Oliwa K, Adley C (2010) An overview of foodborne pathogen detection: in the perspective of biosensors. Biotechnol Adv 28:232–254
Gil ES, Melo GR (2010) Electrochemical biosensors in pharmaceutical analysis. Braz J Pharm Sci 46:375–381
Arora P, Sindhu A, Kaur H, Dilbaghi N, Chaudhury A (2013) An overview of transducers as platform for the rapid detection of foodborne pathogens. Appl Microbiol Biotechnol 97:1829–1836
Wang Y, Duncan TV (2017) Nanoscale sensors for assuring the safety of food products. Curr Opin Biotechnol 44:74–86
Kokkinos C, Economou A, Prodromidis MI (2016) Electrochemical immunosensors: critical survey of different architectures and transduction strategies. Trac Trends Anal Chem 79:88–105
Skladal P (1997) Advances in electrochemical immunosensors. Electroanalysis 9(10):737–745
Skladal P, Kovar D, Krajicek V, Siskova P, Pribyl J, Svabenska E (2013) Electrochemical immunosensors for detection of microorganisms. Int J Electrochem Sci 8(2):1635–1649
Ricci F, Adornetto G, Palleschi G (2012) A review of experimental aspects of electrochemical immunosensors. Electrochim Acta 84:74–83
Carvalhal RF, Kubota LT, Freire RS (2005) Polycrystalline gold electrodes: a comparative study of pretreatment procedures used for cleaning and thiol self-assembly monolayer formation. Electroanalysis 17(14):1251–1259
Pimenta-Martins MGR, Furtado RF, Heneine LGD, Dias RS, Borges MD, Alves CR (2012) Development of an amperometric immunosensor for detection of staphylococcal enterotoxin type a in cheese. J Microbiol Methods 91:138–143
Salam F, Tothill IE (2009) Detection of Salmonella typhimurium using an electrochemical immunosensor. Biosens Bioelectron 24(8):2630–2636
Babacan S, Pivarnik P, Letcher S, Rand AG (2000) Evaluation of antibody immobilization methods for piezoelectric biosensor application. Biosens Bioelectron 15(11–12):615–621
Danczyk R, Krieder B, North A, Webster T, HogenEsch H, Rundell A (2003) Comparison of antibody functionality using different immobilization methods. Biotechnol Bioeng 84(2):215–223
Cheng C, Peng Y, Bai J, Zhang X, Liub Y, Fan X, Ning B, Gao Z (2014) Rapid detection of listeria monocytogenes in milk by self-assembled electrochemical immunosensor. Sensors Actuators B 190:900–906
Luczak T, Osinska M (2017) New self-assembled layers composed with gold nanoparticles, cysteamine and dihydrolipoic acid deposited on bare gold template for highly sensitive and selective simultaneous sensing of dopamine in the presence of interfering ascorbic and uric acids. J Solid State Electrochem 21(3):747–758
Yang Z, Gonzalez-Cortes A, Jourquin G, Viré J-C, Kauffmann J-M, Delplancke J-L (1995) Analytical application of self-assembled monolayers on gold electrodes: critical importance of surface pretreatment. Biosens Bioelectron 10(9):789–795
Sun X, Zhu Y, Wang XY (2011) Amperometric Immunosensor based on a protein a/deposited gold nanocrystals modified electrode for Carbofuran detection. Sensors 11(12):11679–11691
Salmain M, Ghasemi M, Boujday S, Pradier CM (2012) Elaboration of a reusable immunosensor for the detection of staphylococcal enterotoxin a ( SEA) in milk with a quartz crystal microbalance. Sensors Actuators B Chem 173:148–156
Derkus B, Emregul K, Mazi H, Emregul E, Yumak T, Sinag A (2014) Protein a immunosensor for the detection of immunoglobulin G by impedance spectroscopy. Bioprocess Biosyst Eng 37(5):965–976
Skottrup PD, Nicolaisen M, Justesen AF (2008) Towards on- site pathogen detection using antibody- based sensors. Biosens Bioelectron 24(3):339–348
Green AA, Hughs WL (1955) Methods in enzymology, vol v. 1. Academic Press, New York
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Avrameas S (1969) Coupling of enzymes to proteins with glutaraldehyde: Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 6:43–52
Susmel S, Sullivan CK, Guilbault GG (2000) Human cytomegalovirus detection by a quartz crystal microbalance immunosensor. Enzym Microb Technol 27(9):639–645
Tlili A, Abdelghani A, Hleli S, Maaref MA (2004) Electrical characterization of a thiol SAM on gold as a first step for the fabrication of immunosensors based on a quartz crystal microbalance. Sensors 4(6–7):105–114
Dijksma M, Boukamp BA, Kamp B, van Bennekom WP (2002) Effect of hexacyanoferrate(ii/iii) on self-assembled monolayers of thioctic acid and 11-mercaptoundecanoic acid on gold. Langmuir 18:3105–3112
Jung C, Dannenberger O, Xu Y, Buck M, Grunze M (1998) Self-assembled monolayers from organosulfur compounds: a comparison between sulfides, disulfides, and thiols. Langmuir 14:1103–1107
Leopold MC, Bowden EF (2002) Influence of gold substrate topography on the voltammetry of cytochrome c adsorbed on carboxylic acid terminated self-assembled monolayers. Langmuir 18:2239–2245
Mantzila AG, Maipa V, Prodromidis MI (2008) Development of a faradic impedimetric immunosensor for the detection of Salmonella typhimurium in milk. Anal Chem 80(4):1169
Droz E, Taborelli M, Descouts P, Wells TNC, Werlen RC (1996) Covalent immobilization of immunoglobulins G and fab’ fragments on gold substates for scanning force microscopy imaging in liquids. J Vac Sci Technol B 14:1422–1426
Björk I, Petersson B, Sjöquist J (1972) Some physicochemical properties of protein a from Staphylococcus Aureus. Eur J Biochem 29(3):579–584
Gopinath SCB, Tang TH, Citartan M, Chen Y, Lakshmipriya T (2014) Current aspects in immunosensors. Biosens Bioelectron 57:292–302
Furtado RF, Alves CR, Moreira ACO, Azevedo RM, Dutra RF (2012) A novel xyloglucan film-based biosensor for toxicity assessment of ricin in castor seed meal. Carbohydr Polym 89:586–591
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta 1767:1073–1101
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39(8):549–559
Mehrvar M, Abdi M (2004) Recent developments, characteristics, and potential applications of electrochemical biosensors. Anal Sci 20:1113–1126
Rosatto SS, Freire RS, Durán N, Kubota LT (2001) Amperometric biosensors for phenolic compounds determination in the environmental interess samples. Química Nova 24:77–86
Lei C-X, Hu S-Q, Gao N, Shen G-L, Yu R-Q (2004) An amperometric hydrogen peroxide biosensor based on immobilizing horseradish peroxidase to a nano-au monolayer supported by sol–gel derived carbon ceramic electrode. Bioelectrochemistry 65:33–39
Oh B, Kim Y, Park K, Lee W, Choi JW (2004) Surface plasmon resonance immunosensor for the detection of Salmonella typhimurium. Biosens Bioelectron 19(11):1497–1504
Oh BK, Lee W, Lee WH, Choi JW, Kim YK (2004) Surface plasmon resonance immunosensor using self-assembled protein G for the detection of Salmonella paratyphi. J Biotechnol 111:1–8
Bae Y, Park K, Oh B, Lee W, Choi JW (2005) Immunosensor for detection of Salmonella typhimurium based on imaging ellipsometry. Colloids Surf A Physicochem Eng Asp 257:19–23
O’Shannessy DJ, Brigham-Burke M, Peck K (1992) Immobilization chemistries suitable for use in the BlAcore surface Plasmon resonance detector. Anal Biochem 205:132–136
Barie N, Rapp M (2001) Covalent bound sensing layers on surface acoustic wave (SAW) biosensors. Biosens Bioelectron 16:979–987
Lee KM, Runyon M, Herrman TJ, Hsieh J, Phillips R (2015) Review of Salmonella detection and identification methods: aspects of rapid emergency response and food safety. Food Control 47:264–276
Knirel YA, Kocharova NA, Bystrova OV, Katzenellenbogen E, Gamian A (2002) Structures and serology of the O-specific polysaccharides of bacteria of the genus Citrobacter. Arch Immunol Ther Exp 50(6):379–391
Péterfi Z, Kustos I, Kilár F, Kocsis B (2007) Microfluidic chip analysis of outer membrane proteins responsible for serological cross-reaction between three gram-negative bacteria: Proteus Morganii O34, Escherichia Coli O111 and Salmonella Adelaide O35. J Chromatogr A 1155(1):214–217
Kim YS, Raston NHA, Gu MB (2016) Aptamer-based nanobiosensors. Biosens Bioeletron 76:2–19
Acknowledgements
The authors would like to thank the Brazilian agencies, CNPq, FUNCAP, and CAPES, for their financial support, Embrapa Tropical Agroindustry and National Center of Energy and Materials Research (CNPEM). Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melo, A.M.A., Alexandre, D.L., Oliveira, M.R.F. et al. Optimization and characterization of a biosensor assembly for detection of Salmonella Typhimurium. J Solid State Electrochem 22, 1321–1330 (2018). https://doi.org/10.1007/s10008-017-3767-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3767-0