Abstract
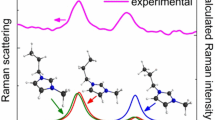
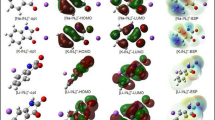
The structural, electronic, and topological properties of a series of four members of potassium 6-X-2-isonicotinoyltrifluoroborate (X=H, F, Cl, Br) salts have been explored by using ab initio calculations with the hybrid B3LYP/6-311++G** method. According to the potential energy surface only the properties for the most stable conformer of each member of the series and their anions were analyzed in function of electronegativity and atomic radius of X. The results show that when X=H, the salt and its anion have symmetry CS while the symmetry change to C1 for the halogenated F, Cl, and Br derivatives and their anions. Both, electronegativity and atomic radius properties show higher effects on V than on μ. Similar behaviors are observed when the Mulliken charges on N and X atoms are analyzed vs electronegativites, and atomic radius of X while an important decreasing on NPA charges of X is observed when increase its electronegativity. The strong influence of electronegativity and atomic radius of X are evidenced in the low bond order value observed in the C1 atom of F salt. The strong energetic π*C2-C3 → π*C4-C5 transition observed only for the F salt confer to it a high stability. The frontier orbitals have revealed that the 6-H-IFTB salt is the less reactive species while the higher reactivity is predicted for the Br salt. Evidently, the smaller electronegativity and higher atomic radius of Br justify the high reactivity predicted for its salt.

Exploring properties of potasium 6-X-2-isonicotinoyltrifluoroborate (X= H, F, Cl, Br)










Similar content being viewed by others
References
Falcicchio A, Nilsson Lill SO, Perna FM, Salomone A, Coppi DI, Cuocci C, Stalked D, Capriati V (2015) Organotrifluoroborates as attractive self-assembling systems: the case of bifunctional dipotassium phenylene-1,4bis(trifluoroborate). Dalton Trans 44:19447–19450
Kamiński R, Jarzembska KN, Dąbrowski M, Durka K, Kubsik M, Serwatowski J, Woźniak K (2016) Finding rules governing layered architectures of trifluoroborate potassium salts in the solid state. Cryst Growth Des 16(3):1687–1700
Bateman CM, Zakharov LN, Abbey ER (2017) Crystal structure of triphenylphosphoniummethylenetrifluoroborate. Acta Cryst E73:1140–1142
Iramain MA, Davies L, Brandán SA (2018) FTIR, FT-Raman and UV-visible spectra of Potassium 3-furoyltrifluoroborate. J Mol Struct 1158:245–254
Iramain MA, Davies L, Brandán SA (2018) Evaluating structures, properties and vibrational and electronic spectra of the potassium 2-isonicotinoyltrifluoroborate salt. J Mol Struct 1163:41–53
Iramain MA, Davies L, Brandán SA (2019) Structural and spectroscopic differences among the Potassium 5-hydroxypentanoyltrifluoroborate salt and the furoyl and isonicotinoyl salts. J Mol Struct 1176:718–728
Iramain MA, Davies L, Brandán SA. Spectroscopic FT-IR, FT-Raman and Ultravioleta-visible studies of the Potassium 5-hydroxypentanoyltrifluoroborate salt, presented in European Congress of Molecular Spectroscopy (EUCMOS Congress), Coimbra, Portugal, August 2018.
Iramain MA, Ledesma AE, Brandán SA (2018) Structural properties and vibrational analysis of Potassium 5-Br-2-isonicotinoyltrifluoroborate salt. Effect of Br on the isonicotinoyl ring. J Mol Struct
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A38:3098–3100
Miertus S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. Chem Phys 55:117–129
Tomasi J, Persico J (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B113:6378–6396
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516
Chain F, Romano E, Leyton P, Paipa C, Catalán CAN, Fortuna MA, Brandán SA (2014) An experimental study of the structural and vibrational properties of sesquiterpene lactone cnicin using FT-IR, FT-Raman, UV–visible and NMR spectroscopies. J Mol Struct 1065-1066:160–169
Minteguiaga M, Dellacassa E, Iramain MA, Catalán CAN, Brandán SA (2017) A structural and spectroscopic study on carquejol, a relevant constituent of the medicinal plant Baccharis trimera (Less.) DC. (Asteraceae). J Mol Struct 1150:8–20
Minteguiaga M, Dellacassa E, Iramain MA, Catalán CAN, Brandán SA (2018) Synthesis, spectroscopic characterization and structural study of 2-isopropenyl-3-methylphenol, carquejiphenol, a carquejol derivative with potential medicinal use. J Mol Struct 1165:332–343
Romani D, Brandán SA, Márquez MJ, Márquez MB (2015) Structural, topological and vibrational properties of an isothiazole derivatives series with antiviral activities. J Mol Struct 1100:279–289
Chain F, Ladetto MF, Grau A, Catalán CAN, Brandán SA (2016) Structural, electronic, topological and vibrational properties of a series of N-benzylamides derived from Maca (Lepidium meyenii) combining spectroscopic studies with ONION calculations. J Mol Struct 1105:403–414
Nielsen AB, Holder AJ (2000-2003) GaussView, User’s Reference. GAUSSIAN, Inc., Pittsburgh
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford
Gledening ED, Badenhoop JK, Reed AD, Carpenter JE, Weinhold FF (1996) NBO 3.1. Theoretical Chemistry Institute, University of Wisconsin, Madison
Biegler-Köning F, Schönbohm J, Bayles D, AIM2000 (2001) A program to analyze and visualize atoms in molecules. J Comput Chem 22:545
Berionni G, Maji B, Knochel P, Mayr H (2012) Nucleophilicity parameters for designing transition metal-free C–C bond forming reactions of organoboron compounds. Chem Sci 3:878–882
Ugliengo P (1998) Moldraw Program. University of Torino, Dipartimento Chimica IFM, Torino
Mootz D, Wussow HG (1981) Crystal structures of pyridine and pyridine trihydrate. J Chem Phys 75(3)
Schpector JZ, Guadagnin RC, Stefani HA, do Canto Visentin L (2008) Potassium trifluoro[(Z)-3-methoxyprop-1-enyl]borate. Acta Crystallogr E Acta Cryst E64:m1525
Bader RFW (1990) Atoms in molecules. a quantum theory. Oxford University Press, Oxford, ISBN: 0198558651
Poater J, Solà M, Bickelhaupt FM (2006) Hydrogen–hydrogen bonding in planar biphenyl, predicted by atoms-in-molecules theory, does not exist. Chem Eur J 12:2889–2895
Poater J, Solà M, Bickelhaupt FM (2006) A model of the chemical bond must be rooted in quantum mechanics, provide insight, and possess predictive power. Chem Eur J 12:2902–2905
Funding
This work was supported with grants from CIUNT Project 26/D608 (Consejo de Investigaciones, Universidad Nacional de Tucumán).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Rights and permissions
About this article
Cite this article
Iramain, M.A., Brandán, S.A. Exploring properties of potassium 6-X-2-isonicotinoyltrifluoroborate (X=H, F, Cl, Br) salts and their anions by using ab initio calculations. J Mol Model 25, 348 (2019). https://doi.org/10.1007/s00894-019-4234-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4234-x