Abstract
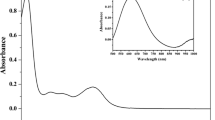
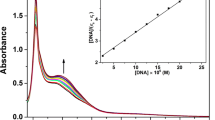
A histidine-2′-deoxyguanosine hybrid, Phac-Hse(p5′dG)-His-OH (I), was synthesized, and its reaction with cisplatin was monitored by reversed-phase HPLC and 1H NMR. Two new compounds, II and III, were observed to be simultaneously formed, II in larger proportion than III. These products were isolated after HPLC purification and extensively characterized. Both II and III contained platinum, had the same mass and showed a bathochromic displacement of their absorption maxima with respect to that of I. Both remained undegraded upon enzymatic digestion and yielded I when treated with NaCN. From these data and the information provided by 1H NMR analysis, we inferred that II and III were constitutional isomers, in particular chelates in which platinum was coordinated to the N7 of guanine and either the Nπ or the Nτ imidazole nitrogens, respectively. No preference of the metal for either of these N-donors was observed.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 3 May 1999 / Accepted: 24 August 1999
Rights and permissions
About this article
Cite this article
Beltrán, M., Onoa, G., Pedroso, E. et al. Study of the interaction between a histidine-deoxyguanosine hybrid and cisplatin. JBIC 4, 701–707 (1999). https://doi.org/10.1007/s007750050342
Issue Date:
DOI: https://doi.org/10.1007/s007750050342