Abstract
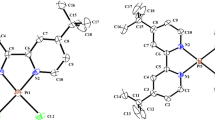
Cu(II) and Zn(II) complexes with Schiff bases derived from diethylenetriamine, [Cu(C22H23N5)(H2O)(ClO4)2] (1), [Cu(C14H18N4)(ClO4)](ClO4) (2), and [Zn(C10H17N5Cl)]4[ZnCl4]2·H2O (3), have been synthesized and structurally characterized. The interactions of the complexes with CT-DNA were investigated by electronic absorption, circular dichroism (CD), and fluorescence spectroscopies. All three complexes appear to bind to DNA via groove binding modes. The order of DNA binding strength was 2 > 1 > 3. The complexes proved to be capable of efficient cleavage of pBR322 DNA at micromolar concentrations in the presence of ascorbic acid as a reducing regent. The hydroxyl radical may be the reactive species, and H2O2 may be involved in DNA strand breakage induced by these complexes. Furthermore, in vitro cytotoxicities of the complexes against four human carcinoma cell lines (HepG2, MGC-803, EC9706, and MCF-7) were screened by MTT assays. Complex 2 shows potent activity against HepG2 and MGC-803 cell lines; for all four cell lines, the activities of the complexes follow the order: 2 > 1 > 3. Hence, the different anticancer activities of the complexes may be correlated with their DNA binding abilities.












Similar content being viewed by others
References
Medici S, Peana M, Nurchi VM, Lachowicz JI, Crisponi G, Zoroddu MA (2015) Coord Chem Rev 284:329–350
Wilson JJ, Lippard SJ (2014) Chem Rev 114(8):4470–4495
Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP (2008) Cancer Treat Rev 34(4):368–377
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Chem Rev 114(1):815–862
Vieira AP, Wegermann CA, Da Costa Ferreira AM (2018) New J Chem 42(15):13169–13179
Dìez M, Cerdàn FJ, Arroyo M, Balibrea JL (1989) Cancer 63(4):726–730
Nayak SB, Bhat VR, Upadhyay D, Udupa SL (2003) Indian J Physiol Pharmacol 47(1):108–110
Tisato F, Marzano C, Porchia M, Pellei M, Santini C (2010) Med Res Rev 30(4):708–749
Erxleben A (2018) Coord Chem Rev 360:92–121
Hangan AC, Stan RL, Turza A, Oprean LS, Páll E, Gheorghe CS, Sevastre B (2017) Transit Metal Chem 42(2):153–164
Mardani Z, Kazemshoar DR, Moeini K, Hajabbas FA, Carpenter WC, Slawin AMZ, Woollinsd JD (2018) RSC Adv 8(50):28810–28824
Stacy AE, Palanimuthu D, Bernhardt PV, Kalinowski DS, Jansson PJ, Richardson DR (2016) J Med Chem 59(10):4965–4984
Gümüş F, Eren G, Açık L, Çelebi A, Öztürk F, Yılmaz Ş, Saǧkan RI, Gür S, Özkul A, Elmalı A, Elerman Y (2009) J Med Chem 52(5):1345–1357
Alpaslan G, Boyacioglu B, Demir N, Tümer Y, Yapar G, Yıldırım N, Yıldız M, Ünver H (2019) J Mol Struct 1180:170–178
Saghatforoush L, Moeini K, Hosseini YSA, Mardani Z, Hajabbas FA, Jameson HT, Telfer SG, Woollins JD (2018) RSC Adv 8(62):35625–35639
Zaltariov MF, Hammerstad M, Arabshahi HJ, Jovanović K, Richter KW, Cazacu M, Shova S, Balan M, Andersen NH, Radulović S, Reynisson J, Andersson KK, Arion VB (2017) Inorg Chem 56(6):3532–3549
Zhou XQ, Li Y, Zhang DY, Nie Y, Li ZJ, Gu W, Liu X, Tian JL, Yan SP (2016) Eur J Med Chem 114:244–256
Bai YL, Zhang YW, Xiao JY, Guo HW, Liao XW, Li WJ, Zhang YC (2018) Transit Metal Chem 43(2):171–183
Kumar P, Gorai S, Santra MK, Mondal B, Manna D (2012) Dalton Trans 41(25):7573–7581
Sheldrick GK (1996) SHELXTL reference manual. Siemens Analytical X-ray Systems Inc., Madison
Wolfe A, Shimer GH, Meehan T (1987) Biochemistry 26(20):6392–6396
Pasternack RF, Caccam M, Keogh B, Stephenson TA, Williams AP, Gibbs EJ (1991) J Am Chem Soc 113(18):6835–6840
Boča M, Valko M, Kickelbick G, Ďurı́k M, Linert W (2003) Inorg Chim Acta 349:111–122
Borge VV, Patil RM (2019) Microchem J 145:456–459
Emara AAA (2010) Spectrochim Acta A 77(1):117–125
Shit S, Sasmal A, Dhal P, Rizzoli C, Mitra S (2016) J Mol Struct 1108:475–481
Mancha MK, Gurumoorthy P, Arul AS, Ramalakshmi N (2017) J Mol Struct 1143:478–486
Kumar S, Pal Sharma PR, Venugopalan P, Singh GV, Chhibber S, Aree T, Witwicki M, Ferretti V (2018) Inorg Chim Acta 469:288–297
Hasan MA, Kumari N, Singh K, Singh K, Mishra L (2016) Spectrochim Acta A 152:208–217
Fu XB, Liu DD, Lin Y, Hu W, Mao ZW, Le XY (2014) Dalton Trans 43(23):8721–8737
Sirajuddin M, Ali S, Badshah A (2013) J Photochem Photobiol B 124:1–19
Liu YX, Mo HW, Lv ZY, Shen F, Zhang CL, Qi YY, Mao ZW, Le XY (2018) Transit Metal Chem 43(3):259–271
Tjioe L, Meininger A, Joshi T, Spiccia L, Graham B (2011) Inorg Chem 50(10):4327–4339
Protas AV, Popova EA, Mikolaichuk OV, Porozov YB, Mehtiev AR, Ott I, Alekseev GV, Kasyanenko NA, Trifonov RE (2018) Inorg Chim Acta 473:133–144
Raja DS, Bhuvanesh NSP, Natarajan K (2011) Inorg Chem 50(24):12852–12866
Deng J, Su G, Chen P, Du Y, Gou Y, Liu Y (2018) Inorg Chim Acta 471:194–202
Douglas K (1996) Transit Metal Chem 21(5):474–480
Rangasamy L, Sethu R, Mani G, Nattamai SPB, Mallayan P, Anvarbatcha R, Mohamad AA (2015) Dalton Trans 44:10210–10227
Lian WJ, Wang XT, Xie CZ, Tian H, Song XQ, Pan HT, Qiao X, Xu JY (2016) Dalton Trans 45:9073–9087
Ramakrishnan S, Palaniandavar M (2008) Dalton Trans 29:3866–3878
Lee WY, Yan YK, Lee PP, Tan SJ, Lim KH (2012) Metallomics 4(2):188–196
Gaetke LM, Chow CK (2003) Toxicology 189(1):147–163
Acknowledgements
The authors acknowledge financial assistance from Hunan Province Key Research and Development Project of China (2017SK2254).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, Z., Yan, J., Yu, W. et al. Three Schiff base complexes based on diethylenetriamine: synthesis, structure, DNA binding and cleavage, and in vitro cytotoxicity. Transit Met Chem 44, 463–474 (2019). https://doi.org/10.1007/s11243-019-00327-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-019-00327-1