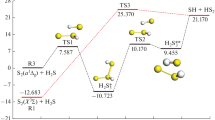
Abstract
The oxidation of sulfite to sulfate by two different models of the active site of sulfite oxidase has been studied. Both protonated and deprotonated substrates were tested. Geometries were optimized with density functional theory (TPSS/def2-SV(P)) and energies were calculated either with hybrid functionals and large basis sets (B3LYP/def2-TZVPD) including corrections for dispersion, solvation, and entropy, or with coupled-cluster theory (LCCSD(T0)) extrapolated toward a complete basis set. Three suggested reaction mechanisms have been compared and the results show that the lowest barriers are obtained for a mechanism where the substrate attacks a Mo-bound oxo ligand, directly forming a Mo-bound sulfate complex, which then dissociates into the products. Such a mechanism is more favorable than mechanisms involving a Mo–sulfite complex with the substrate coordinating either by the S or O atom. The activation energy is dominated by the Coulomb repulsion between the Mo complex and the substrate, which both have a negative charge of −1 or −2.













Similar content being viewed by others
References
Kaim W, Schwederski B (1991) Bioinorganic chemistry: inorganic elements in the chemistry of life J. Wiley, Chichester
Hu YL, Ribbe MW (2010) Acc Chem Res 43:475
Hille R (1996) Chem Rev 96:2757
Mendel RR, Bittner F (2006) Biochim Biophys Acta 1763:621–663
Feng CJ, Tollin G, Enemark JH (2007) Biochim Biophys Acta 1774:527–539
Mendel RR (2007) J Exp Bot 58:2289–2296
Pilato RS, Stiefel EI (1999) Bioinorganic catalysis, 2nd edn (Reedijk J, Bouwman E eds) Marcel Dekker, New York, pp 81–152
Kisker C, Schindelin H, Pacheco A, Wehbi WA, Garrett RM, Rajagopalan KV, Enemark JH, Rees DC (1997) Cell 91:973–983
Schrader N, Fischer K, Theis K, Mendel RR, Schwarz G, Kisker C (2003) Structure 11:1251–1263
Kappler U, Bailey S (2005) J Biol Chem 280:24999–25007
Karakas E, Wilson HL, Graf TN, Xiang S, Jaramillo-Buswuets S, Rajagopalan KV, Kisker C (2005) J Biol Chem 280:33506–33515
Kappler U, Bailey S, Feng CJ, Honeychurch MJ, Hanson GR, Bernhardt PV, Tollin G, Enemark JH (2006) Biochemistry 45:9696–9705
Hille R (1994) Biochem Biophys Acta 1184:143
Hille R (1997) J Biol Inorg Chem 2:804
Das SK, Chaudhury PK, Biswas D, Sarkar S (1994) J Am Chem Soc 116:9061–9070
Chaudhury PK, Das SK, Sarkar S (1996) Biochem J 319:953–959
Lorber C, Plutino MR, Elding LI, Nordlander E (1997) J Chem Soc Dalton Trans 21:3997–4004
Nagarajan K, Chaudhury PK, Srinivasan BR, Sarkar S (2001) Chem Commun 1786–1787
Pal K, Chaudhury PK, Sarkar S (2007) Chem Asian J 2:956–964
George GN, Garrett RM, Graf T, Prince RC, Rajagopalan KV (1998) J Am Chem Soc 120:4522–4523
Klein EL, Raitsimring AM, Atashkin AV, Rajapakshe A, Johnson-Winters K, Arnold AR, Potapov A, Goldfarb D, Enemark JH (2012) Inorg Chem 51:1408–1418
Klein EL, Raitsimring AM, Atashkin AV, Enemark JH (2013) Coord Chem Rev 257:110–118
Astashkin AV, Johnson-Winters K, Klein EL, Feng CJ, Wilson HL, Rajagopalan KV, Raitsimring AK, Enemark JH (2008) J Am Chem Soc 130:8471–8480
Thapper A, Deeth RJ, Nordlander E (1999) Inorg Chem 38:1015–1018
Peariso K, McNaughton RL, Kirk M (2002) J Am Chem Soc 124:9006–9007
Majumdar A, Sarkar S (2011) Coord Chem Rev 255:1039–1054
Hernandez-Marin E, Ziegler T (2009) Inorg Chem 48:1323–1333
Siegbahn PEM, Borowski T (2006) Acc Chem Res 39:729–738
Siegbahn PEM, Himo F (2009) J Biol Inorg Chem 14:643–651
Li J-L, Mata RA, Ryde U (2013) J Chem Theory Comput 9:1799–1807
Liao R-Z, Thiel W (2012) J Chem Theory Comput 8:3793–3803
Liao R-Z, Thiel W (2013) J Comput Chem 34:2389–2397
Startk JG, Wallace HG (1982) Chemistry data book, p 74
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Phys Rev Lett 91:146401
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305
Eichkorn K, Treutler O, Öhm H, Häser M, Ahlrichs R (1995) Chem Phys Lett 240:283–290
Eichkorn K, Weigend F, Treutler O, Ahlrichs R (1997) Theor Chem Acc 97:119–126
Rappoport D, Furche F (2010) J Chem Phys 133:134105
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Becke AD (1993) J Chem Phys 98:5648
Klamt A, Schüürmann J (1993) J Chem Soc Perkin Trans 2(5):799–805
Schäfer A, Klamt A, Sattel D, Lohrenz JCW, Eckert F (2000) Phys Chem Chem Phys 2:2187–2193
Klamt A, Jonas V, Bürger T, Lohrenz JCW (1998) J Phys Chem 102:5074
Sharp KA (1990) Annu Rev Biophys Biophys Chem 19:301
Honig B (1995) Science 268:1144
Treutler O, Ahlrichs R (1995) J Chem Phys 102:346–354
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
http://toc.uni-muenster.de/DFTD3/getd3.html. Accessed 18 June 2013
Miertus S, Scrocco E, Tomasi J (1981) Chem Phys 55:117
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3093
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA Gaussian 03, revision D.01. Gaussian Inc., Wallingford
Ryde U, Mata RA, Grimme S (2011) Dalton Trans 40:11176–11183
Schütz M, Hetzer G, Werner H-J (1999) J Chem Phys 111:5691
Hampel C, Werner H-J (1996) J Chem Phys 104:6286
Kendall RA, Dunning TH, Harrison RJ (1992) J Chem Phys 96:6796
Dunning TH Jr (1989) J Chem Phys 90:1007–1023
Peterson KA, Figgen D, Dolg M, Stoll H (2007) J Chem Phys 126:124101
Polly R, Werner H-J, Manby FR, Knowles PJ (2004) Mol Phys 102:2311
Werner H-J, Manby FR, Knowles PJ (2003) J Chem Phys 118:8149
Weigend F (2002) Phys Chem Chem Phys 4:4285
Weigend F, Köhn A, Hättig C (2002) J Chem Phys 116:3175
Weigend F (2007) J Comput Chem 29:167
Weigend F, Häser M, Patzelt H, Ahlrichs R (1998) Chem Phys Lett 294:143
Pipek J, Mezey PG (1989) J Chem Phys 90:4916–4926
Mata RA, Werner H-J (2007) Mol Phys 105:2753–2761
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M et al (2012) Molpro, version 2012.1, a package of ab initio programs. see http://www.molpro.net
Helgaker T, Klopper W, Koch H, Noga J (1997) J Chem Phys 106:9639
Harris HH, George GN, Rajagopalan KV (2006) Inorg Chem 45:493–494
Amzel LM (1997) Proteins Struct Funct Genet 28:144
Rulíšek L, Jensen KP, Lundgren K, Ryde U (2006) J Comput Chem 27:1398–1414
Irudayam SJ, Henchman RH (2009) J Phys Chem B 113:5871–5884
Schutz CN, Warshel A (2001) Proteins 44:400–417
Ryde U (1996) Eur Biophys J 24:213–221
Acknowledgments
This investigation has been supported by grants from the Swedish research council (project 2010-5025), from the Swedish Institute, the Crafoord Foundation, the National Basic Research Program of China (973 Program, 2012CB932800), the National Natural Science Foundation of China (NSFC 21103064), and from COST through Action CM1003. It has also been supported by computer resources of Lunarc at Lund University. The collaboration between the Universities of Lund and Göttingen has been carried out within the framework of the International Research Training Group “Metal Sites in Biomolecules—Structures, Regulation, Mechanisms” and M. A. is supported through a Ph.D. scholarship in this International Research Training Group.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Severen, MC., Andrejić, M., Li, J. et al. A quantum-mechanical study of the reaction mechanism of sulfite oxidase. J Biol Inorg Chem 19, 1165–1179 (2014). https://doi.org/10.1007/s00775-014-1172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-014-1172-z