Abstract
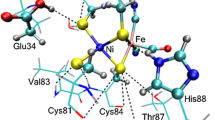
The coordination chemistry of the structural zinc ion in horse liver alcohol dehydrogenase has been examined by quantum chemical geometry optimisations. It is shown that all four cysteine ligands are deprotonated in the enzyme, not only two of them as has been suggested. The Zn-S bond lengths are very sensitive to the theoretical treatment; in vacuum they are predicted to be 15 pm longer than in the crystal structure. Half of this discrepancy is due to electronic correlation, the rest can be attributed to screening of the negative sulphide charges by the enzyme, in particular by N-H-S hydrogen bonds. The potential surface is rather flat, so the large difference in geometry between the crystal and the vacuum structure corresponds to an energy change of less than 35 kJ/mol. The experimental bond lengths can be reproduced only with methods that account explicitly for the enzyme. A dielectric continuum model gives bond lengths which are too long, indicating that the enzyme solvates the coordination sphere better than water. Thus, the structural zinc ion can be used as a sensitive test of methods which try to model the surrounding medium in quantum chemical computations.
Similar content being viewed by others
References
Adman ET, Watenpaugh KD, Jensen LH (1975) NH-S hydrogen bonds in Peptococcus aerogenes ferredoxin, Clostridium pasteurianum rubredoxin, and Chromatium high potential iron protein. Proc Natl Acad Sci USA 72: 4854–4858
Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C (1989) Electronic structure calculations on workstation computers: The program system Turbomole. Chem Phys Lett 162: 165–169
Al-Karadaghi S, Cedergren-Zeppezauer ES, Petramos K, Hovmöller S, Terry H, Dauter Z, Wilson KS (1994) Refined crystal structure of liver alcohol dehydrogenase-NADH complex at 1.8 Å resolution. Acta Crystallogr D50: 793–807
Al-Karadaghi S, Cedergren-Zeppezauer ES, Dauter Z, Wilson KS (1995) Refined crystal structure of Cu-substituted alcohol dehydrogenase at 2.1 Å resolution. Acta Crystallogr D51: 805–813
Andersson K, Blomberg MRA, Fülscher MP, Kellö V, Lindh R, Malmqvist P-Å, Noga J, Olsen J, Roos BO, Sadlej AJ, Siegbahn PEM, Urban M, Widmark P-0 (1994) Molcas version 3. University of Lund, Sweden
Brändén C-I, Tooze J (1991) Introduction to protein structure. Garland Inc., New York, pp 113–285
Cedergren-Zeppezauer ES, Andersson I, Ottonello S, Bignetti E (1985) X-ray analysis of structural changes induced by reduced nicotinamide adenine dinucleotide when bound to cysteine-46-carboxymethylated liver alcohol dehydrogenase. Biochemistry 24: 4000–4010
Chakrabarti P (1989) Geometry of interaction of metal ions with sulfur-containing ligands in protein structures. Biochemistry 28: 6081–6085
Cotton FA, Wilkinson G (1988) Advanced inorganic chemistry. Wiley, New York
Eklund H, Brändén C-I (1983) The role of zinc in alcohol dehydrogenase. In: Spiro TG (ed) Zinc enzymes. John Wiley & Sons, New York, pp 124–153
Eklund H, Samama J-P, Wallén L, Brändén C-I (1981) Structure of a triclinic ternary complex of horse-liver alcohol dehydrogenase at 2.9 Å resolution. J Mol Biol 146: 561–587
Fogarasi G, Zhou X, Taylor PW, Pulay P (1992) The calculation of ab initio molecular geometries: efficient optimization by natural internal coordinates and empirical correction by offset forces. J Am Chem Soc 114: 8191–8201
Fraústo da Silvia JJR, Williams RJP (1991) The Biological Chemistry of the Elements. Clarendon Press, Oxford, pp 182–184
Gartner DR, Krauss M (1993) Ab initio quantum chemical study of the cobalt d-d spectroscopy of several substituted zinc enzymes. J Am Chem Soc 115: 10247–10257
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley-Interscience, New York, pp 251–260
Irish DE, McCaroll B, Young TF (1963) Raman study of zinc chloride solutions. J Chem Phys 39: 3436–3444
Langhoff SR, Bauschlicher CW (1988) Ab initio studies of transition metal systems. Annu Rev Phys Chem 39: 181–212
Lüthi HP, Siegbahn PEM, Almlöf J (1985) The effect of electron correlation on the metal-ligand interaction in Fe(CO)5. J Phys Chem 89: 2156–2161
Mikkelsen KV, Ågren H, Jensen HJA, Helgaker T (1988) A multiconfigurational self-consistent reaction-field method. J Chem Phys 89: 3086–3095
Pearlman DA, Case DA, Caldwell JC, Seibel GL, Singh UC, Weiner P, Kollman PA (1991) Amber 4.0, University of California, San Francisco, USA
Pettersson G (1987) Liver alcohol dehydrogenase. CRC Crit Rev Biochem 21: 349–389
Rice JE, Horn H, Lengsfiels BH, McLean AD, Carter JT, Replogle ES, Barnes LA, Maluendes SA, Lie GC, Gutwski M, Rude WE, Sauer SPA, Lindh R, Andersson K, Chevalier TS, Widmark P-O, Bouzida D, Pacansky G, Singh K, Gillan CJ, Carnevali P, Swope WC, Liu B (1995) Mulliken™ Version 2.18d, internal release. IBM Corporation,Almaden, USA
Ryde U (1994) The coordination chemistry of the catalytic zinc ion in alcohol dehydrogenase studied by ab initio quantum chemical calculations. Int J Quant Chem 52: 1229–1243
Ryde U (1995a) Molecular dynamics simulations of alcohol dehydrogenase with a four- or five-coordinate catalytic zinc ion. Proteins: Struct Funct Genet 21: 40–56
Ryde U (1995b) On the role of Glu-68 in alcohol dehydrogenase. Prot Sci 4: 1124–1132
Ryde U (1996) The coordination of the catalytic zinc ion in alcohol dehydrogenase studied by combined quantum chemical and molecular mechanical calculation. J Comput-Aided Molec Design. (in press)
Schäfer A, Horn H, Ahlrichs R (1992) Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J Chem Phys 97: 2571–2577
Singh UC, Kollman PA (1986) A combined ab initio quantum mechanical and molecular mechanical method for carrying out simulations on complex molecular systems. J Comp Chem 7: 718–730
Sharp KA, Honig B (1990) Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Biophys Chem 19: 301–332
Tapia O, Goscinski O (1975) The self-consistent reaction field theory of solvent effects. Mol Phys 29: 1653–1661
Teleman O, Jönsson B (1986) Vectorizing a general purpose molecular dynamics simulation program. J Comp Chem 7: 58–66
Tossell JA (1991) Calculations of the structures, stabilities, and Raman and Zn NMR spectra of ZnCln(OH2)a 2−n species in aqueous solution. J Phys. Chem 95: 366–371
Warshel A (1991) Computer modeling of chemical reactions in enzymes and solutions. J Wiley & Sons, New York, pp 209–211
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ryde, U. The coordination chemistry of the structural zinc ion in alcohol dehydrogenase studied by ab initio quantum chemical calculations. Eur Biophys J 24, 213–221 (1996). https://doi.org/10.1007/BF00205102
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00205102