Abstract
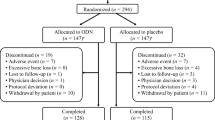
We conducted a post-marketing observational study to investigate the safety and effectiveness of eldecalcitol for the treatment of osteoporosis in a Japanese clinical setting. The observation period was 12 months for women and 36 months for men. The final results for the female patients have already been published. In this article, the final results for the male patients are reported. A total of 470 male osteoporosis patients were enrolled. The safety analysis set included 431 patients (mean age, 76.8 years; mean ± SD follow-up period, 631.0 ± 450.3 days), and 175 patients continued treatment throughout the 3-year observational period. Adverse drug reactions (ADRs) were reported in 28 patients (6.49%); the most common ADRs were hypercalcemia (1.16%) and renal impairment (1.16%). Serious ADRs were reported in 5 patients (1.16%). Mean serum calcium was within the normal range throughout the observation period. The cumulative incidence of new vertebral and nonvertebral fractures at 36 months, estimated by Kaplan–Meier analysis, was 10.23 and 4.06%, respectively. At the last observation, mean lumbar spine bone mineral density was 3.49% higher (P < 0.0001) than at baseline, and levels of the bone turnover markers BAP and TRACP-5b were reduced (–14.64%; P = 0.0009, and − 29.51%; P < 0.0001, respectively). In conclusion, the safety and effectiveness of eldecalcitol for the treatment of Japanese male osteoporosis patients was confirmed in clinical practice. Careful monitoring of serum calcium and estimated glomerular filtration rate, both before and during treatment, is necessary to minimize the risk of hypercalcemia and renal impairment while maximizing the effectiveness of eldecalcitol.





Similar content being viewed by others
References
Gullberg B, Johnell O, Kanis JA (1997) World-wide projections for hip fracture. Osteoporos Int 7:407–413
Editorial Committee on Guidelines for Prevention and Treatment of Osteoporosis (2015) Guidelines for prevention and treatment of osteoporosis. Japan Osteoporosis Society, Tokyo, Japan (in Japanese)
Orimo H, Yaegashi Y, Hosoi T, Fukushima Y, Onoda T, Hashimoto T, Sakata K (2016) Hip fracture incidence in Japan: estimates of new patients in 2012 and 25-year trends. Osteoporos Int 27:1777–1784
Xu Z, Fan C, Zhao X, Tao H (2016) Treatment of osteoporosis with eldecalcitol, a new vitamin D analog: a comprehensive review and meta-analysis of randomized clinical trials. Drug Des Devel Ther 10:509–517
Matsumoto T, Miki T, Hagino H, Sugimoto T, Okamoto S, Hirota T, Tanigawara Y, Hayashi Y, Fukunaga M, Shiraki M, Nakamura T (2005) A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 90:5031–5036
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—a randomized, active comparator, double-blind study. Bone 49:605–612
Sakai A, Ito M, Tomomitsu T, Tsurukami H, Ikeda S, Fukuda F, Mizunuma H, Inoue T, Saito H, Nakamura T, e-ADVANCED Study Group (2015) Efficacy of combined treatment with alendronate (ALN) and eldecalcitol, a new active vitamin D analog, compared to that of concomitant ALN, vitamin D plus calcium treatment in Japanese patients with primary osteoporosis. Osteoporos Int 26:1193–1202
Saito H, Kakihata H, Nishida Y, Yatomi S, Nihojima S, Kobayashi Y, Tabata H, Nomura M (2017) The safety and effectiveness profile of eldecalcitol in a prospective, post-marketing observational study in Japanese patients with osteoporosis: interim report. J Bone Miner Metab 35:456–463
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H, Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Ringe JD, Dorst A, Faber H, Ibach K (2004) Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int 24:110–113
Ringe JD, Farahmand P, Faber H, Dorst A (2009) Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int 29:311–315
Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD (2009) Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 24:719–725
Orwoll ES, Binkley NC, Lewiecki EM, Gruntmanis U, Fries MA, Dasic G (2010) Efficacy and safety of monthly ibandronate in men with low bone density. Bone 46:970–976
Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S, HORIZON Recurrent Fracture Trial (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J et al (2012) Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 367:1714–1723
Orwoll ES, Miller PD, Adachi JD, Brown J, Adler RA, Kendler D, Bucci-Rechtweg C, Readie A, Mesenbrink P, Weinstein RS (2010) Efficacy and safety of a once-yearly i.v. infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res 25:2239–2350
Orwoll E, Teglbjærg CS, Langdahl BL, Chapurlat R, Czerwinski E et al (2012) A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab 97:3161–3169
Langdahl BL, Teglbjærg CS, Ho PR, Chapurlat R, Czerwinski E et al (2015) A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab 100:1335–1342
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17
Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, Lindsay R, Mitlak BH (2005) Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 16:510–516
Kondo S, Takano T, Ono Y, Saito H, Matsumoto T (2015) Eldecalcitol reduces osteoporotic fractures by unique mechanisms. J Steroid Biochem Mol Biol 148:232–238
Japanese package insert for Edirol® Capsule. 2016. Online. Available: https://chugai-pharm.jp/hc/ss/pr/drug/edr_cap0075/pi/PDF/edr_pi.pdf. Accessed May, 2017 (in Japanese)
Tomková S, Telepková D, Vanuga P, Killinger Z, Sulková I, Celec P, Payer J (2014) Therapeutic adherence to osteoporosis treatment. Int J Clin Pharmacol Ther 52:663–668
Weycker D, Macarios D, Edelsberg J, Oster G (2006) Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int 17:1645–1652
Kim SY, Schneeweiss S, Liu J, Daniel GW, Chang CL, Garneau K, Solomon DH (2010) Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res Ther 12:R154
Weiss RJ, Wick MC, Ackermann PW, Montgomery SM (2010) Increased fracture risk in patients with rheumatic disorders and other inflammatory diseases: a case-control study with 53,108 patients with fracture. J Rheumatol 37:2247–2250
Fan Y, Wei F, Lang Y, Liu Y (2016) Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int 27:219–228
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a metaanalysis. Osteoporos Int 18:427–444
Gregson CL, Dennison EM, Compston JE, Adami S, Adachi JD et al (2014) Disease-specific perception of fracture risk and incident fracture rates: GLOW cohort study. Osteoporos Int 25:85–95
Acknowledgements
The authors acknowledge the efforts of the physicians and patients who participated in the study. This study was sponsored by Chugai Pharmaceutical Co., Ltd, with considerable support from Taisho Toyama Pharmaceutical Co., Ltd. The authors would like to thank Kokoro Hayashi, PhD, and Rie Ishibashi of inScience Communications, Springer Healthcare (Tokyo, Japan) for providing editorial support, which was funded by Chugai Pharmaceutical Co., Ltd (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors except YK are employees of Chugai Pharmaceutical Co., Ltd. YK is an employee of Taisho Toyama Pharmaceutical Co., Ltd.
About this article
Cite this article
Kondo, S., Kakihata, H., Nishida, Y. et al. The safety and effectiveness profile of eldecalcitol in a prospective, post-marketing observational study in Japanese male patients with osteoporosis. J Bone Miner Metab 37, 292–300 (2019). https://doi.org/10.1007/s00774-018-0915-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-018-0915-2