Abstract
Three new species of the Andean genus Myrosmodes are described: Myrosmodes cleefi Szlach., Mytnik and S. Nowak, Myrosmodes reticulata Szlach., Mytnik and S. Nowak, and Myrosmodes subnivalis Szlach., Mytnik and S. Nowak. The former two species are known from a single population each, from Columbia, and the latter from Columbia and Ecuador. Each species is described and illustrated, and detailed habitat and distribution data are provided. A distribution map of the new species and the distribution range of the genus is presented. A dichotomous key for determination of the Andean species and Myrosmodes is provided. Conservation status assessments are provided for each species; current International Union for Conservation of Nature (IUCN) red list categories and criteria are listed. A brief discussion of endemism in the Andes and Tropical Andes biodiversity hotspot is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Myrosmodes Rchb.f. was described by Reichenbach (1854). Phylogenetic analyses conducted by Álvarez-Molina and Cameron (2009) revealed that Myrosmodes is a sister genus to Aa Rchb.f. and both are closely related to Altensteinia Kunth. These relationships were unresolved for many years, and the taxonomic status of these genera was unclear. Reichenbach filius (1878) suggested that Myrosmodes and Aa are congeneric with Altensteinia. Schlechter (1912) distinguished Aa from Altensteinia again, but he decided to classify Myrosmodes as a synonym of Aa. Half a century later, some taxonomists (e.g., Schweinfurth 1958) considered Altensteinia as a good genus and recognized the other two as its synonyms. In Flora of Ecuador, Garay (1978) revalidated Aa and Myrosmodes, and these three genera are widely accepted up to date (Trujillo and Vargas 2011). All three genera are classified within the subtribe Prescottiinae Dressler (Dressler 1990, 1993; Szlachetko 1995). Myrosmodes is easily distinguishable from two other close relatives, Aa and Altensteinia, by possessing a short peduncle with scarious sheaths, a cucullate lip with fimbriolate margins, an accrescent peduncle anthesis, an inflorescence peduncle that is twice as long or more, flowers growing from the inflorescence apex to its base, and male flowers being distinctly smaller (Trujillo and Vargas 2011).
Myrosmodes includes 15 species, all of them occurring in South American Andes (Fig. 1). The species of Myrosmodes grow at the highest elevations at which an orchid has ever been reported, i.e., almost 5,000 m.a.s.l. (Trujillo and Vargas 2011). The habitats occupied by the Myrosmodes species are moist, freezing, and windy environments of the paramos and the arid conditions of the puna (Rundel 1994). The paramo occurs between the permanent snowline and the upper forest line, so in Andes being at about 3,200–5,000 m.a.s.l. (Josse et al. 2008). Plants of this type of vegetation are adapted to intense ultraviolet radiation, low atmospheric pressure, and drying effects of wind (Luteyn 1992). There are three main types of paramo recognized by physiognomy and structure of vegetation (Cuatrecasas 1968; Harling 1979; Cleef 1981; Ramsay 1992; Jørgensen and Ulloa 1994): the subparamo or shrub paramo, very often dominated by upright and prostrate shrubs; the grass paramo with dominance of tussock grasses with stem rosettes and small patches of shrubs or monotypic, sometimes mixed forest (Ramsay and Oxley 1997; Cuesta and De Bievre 2008); and the superparamo, occurring on the slopes of the highest mountains in Ecuador and Colombia, being divided into two altitudinal belts, the lower and upper superparamo (Cuesta and De Bievre 2008). Puna constitutes some diverse ecosystems covering the high Central Andean Mountains from northern Peru to northern Argentina, above 3,400 m.a.s.l. (Halloy et al. 2008). This type of vegetation is dominated by grasses and shrubs, sometimes halophytic where covering salt plains (García and Beck 2006; Halloy et al. 2008). Some species of Myrosmodes are commonly seen among cushion-forming species of Azorella Lam. (Pridgeon et al. 2003). Anthelme et al. (2011) proved that the nurse plant Azorella may reduce stress reaction caused by harsh environments of the highest ecosystems of Andes.
Map of distribution of the new species on the distribution range of Myrosmodes (circle—Myrosmodes subnivalis, triangle—M. reticulata, square—M. cleefi; distribution range map from Pridgeon et al. 2003)
Mittermeier et al. (2004) suggest that probably near to 10 % of the world’s species of vascular plants occur in tropical Andes and the endemism of the region reaches up to 50–60 % (or higher) of the species. Half of all known orchid species from tropical Andes were described in the last 20 years. Unfortunately, human impact is visibly seen in the decaying diversity of Andean ecosystems. Some regions have almost completely lost their original vegetation; however, some of them are still almost untouched. Paramos and puna are heavily impacted. They are greatly modified by agriculture, grazing, burning, or mining, and puna is also exploited by wood cutting, mostly for fuel. An additional threat is cutting of high Andean forest in Colombia for cultivation of opium puppy. As a result of human pressure in tropical Andes, perhaps only 25 % or less of natural vegetation remained (Mittermeier et al. 2004).
The representatives of Myrosmodes are small terrestrial plants with fleshy, fusiform, fasciculate, and often pubescent roots (Pridgeon et al. 2003). Leaves are small and coriaceous with thick cuticles. Inflorescence is lateral, many-flowered, basipetal, and andromonoecious (Berry and Calvo 1991). Peduncle is short and infundibuliform, accrescent after anthesis; sheaths are scarious. Flowers are nonresupinate, white to greenish-white, subtended by scarious bracts. Sepals are subsimilar and dorsal sepal with petals adnate to the back of the gynostemium. Petals are much narrower than sepals. Lip is cucullate, tubular or flared, with fimbriate margins, often with moniliform hairs and obscure basal calli (Garay 1978; Vargas 1997; Pridgeon et al. 2003). Gynostemium is elongate, erect, rather slender, apically swollen, and dorsiventrally flattened. Conspicuous column part is longer than anther and reduced, rudimentary column foot obliquely adnate to ovary. Anther is erect, ovoid, dorsiventrally flattened, and immovable with two parallel chambers. Pollinia are two, but bipartite, powdery, and oblong-ovoid. Caudiculae are absent. Very large and conspicuous staminodes are wing-like, rather thin. In basal part they are fused with filament and stigma margins, apically being free, almost entirely enclosing anther and crossing each other at apex over anther. Ventral, 3-lobed, confluent, large, transversely elliptic, and flat stigma is surrounded by thick and fleshy collar-like structure. Rostellum is inconspicuous, truncate, and limited to apical portion of stigma median lobe. Single viscidium is relatively small, detachable, cellular, and multilayered (Szlachetko and Rutkowski 2000).
Key to the North Andean species of Myrosmodes:
-
1. Lip subquadrate in outline, middle lobe erect, stiff, oblong, truncate, lip venation strongly thickened, forming a very characteristic net .......... M. reticulata
-
1*. Lip not as above .................... 2
-
2. Ovary long-rostrate at apex, lip distinctly clawed, lamina cordate .................... 3
-
2*. Ovary not rostrate, lip cuneate-flabellate .................... 6
-
3. Rostellum 3-lobed, lip obscurely 3-lobed, middle lobe terminal .......... M. rostratum
-
3*. Rostellum truncate, lip entire, orbicular in outline ...................... 4
-
4. Sepals and petals entire .......... M. rhynchocarpum
-
4*. Sepals and petals erose toward apex ...................... 5
-
5. Petals acute, irregularly dentate near apex, lip papillose inside .......... M. breve
-
5*. Petals obtuse, minutely erose-denticulate near apex, lip glabrous inside .......... M. cochleare
-
6. Sepals dorsally muricate at erose apex, petals erose-denticulate near apex, lip papillose in center: M. ustulatum
-
6*. Sepals dorsally glabrous at apex, petals unevenly lacerate-serrate near apex, lip glabrous in center .................... 7
-
7. Sepals and petals equal in length, anther on free filament .......... M. filamentosum
-
7*. Dorsal sepal and petals shorter than lateral sepals, anther’s filament fused with clinandrium .................... 8
-
8. Lip widest at base, cordate in outline, margins of lateral sepals entire .......... M. nubigenum
-
8*. Lip widest in the middle, lip obtrullate, margins of lateral sepals erose ..................... 9
-
9. Lip with fleshy pads in sinus between the middle and lateral lobes, lateral lobes thickened ........... M. cleefi
-
9*. Lip without fleshy pads in sinus between the middle and lateral lobes, lateral lobes thin .......... M. subnivalis
The studies of pollination of Myrosmodes cochleare Garay conducted by Berry and Calvo (1991) revealed that the species is not pollinated by bumblebees, a frequent pollinator of flowering plants in high mountain range areas, but by calliphorid flies and wasps.
The genus Myrosmodes, as well as other closely related genera of Prescottiinae, have been the subject of ongoing taxonomic work by our team for years. We study herbarium specimens and explore the South American highlands. The collected specimens are determined based on comparison with type material and original descriptions. The taxonomic study on the Colombian collection of orchid specimens deposited in the Herbarium of Universidad Nacional de Colombia in 2012 revealed some new species of Myrosmodes, and therefore, we present them below.
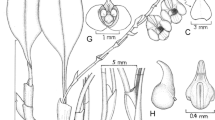
Myrosmodes cleefi Szlach., Mytnik and S. Nowak sp. nov. (Fig. 2)
The species is related to Myrosmodes nubigenum Rchb.f., but the lip is two times larger, widest in the middle, obtrullate in outline, lateral sepals cochleate with erose margins, dorsal sepal oblong-elliptic with keeled apex.
Type: Cleef A.M. 1298-Colombia, Meta, Paramo de Sumapaz, Cerro Nevado del Sumapaz, superparamo, Pico del Nevado, cocas calichosas, flores verdosas, asociado con musgos, alt. 4,300 m (30 Jan 1972), (COL! 180092 holotype).
Terrestrial plants to 7 cm tall, erect, concealed by scarious bracts. Leaves gathered in the basal rosette, several per stem, petiolate; petiole to 1.5 cm long; blade to 2.5 × 1.2 cm, ovate to ovate-lanceolate, subobtuse. Inflorescence 4 cm long, very dense, conical, many-flowered. Flowers green. Floral bracts 15 mm long, flabellate from the cuneate lower part, margins undulate, erose, and undulate. Ovary 8 mm long, glabrous. Sepaline tube 3 mm long. Dorsal sepal 5 × 3 mm, oblong-elliptic, concave, 1-nerved, keeled outside at the apex. Petals 4.8 × 1.3 mm, lanceolate, falcate, acuminate, 1-nerved, with several, irregular fimbriae along margins. Lateral sepals 8–9 × 4.5 mm, obliquely oblong-elliptic, obtuse, concave, erose on margins. Lip 9–10 mm long in total, apically 3-lobed, obtrullate in outline, widest near the middle, with two small, globose, mamilliform thickenings at the base, nerves 7, branching; the middle lobe 1.8 × 1.6 mm, oblong-ovate, obtuse, thick, fimbriate on margins; the lateral lobes much thickened, fimbriate on margins, crossing each other in natural position; sinus between lobes with thick, fleshy pads.
Ecology: terrestrial in superparamo, among mosses; altitude: 4,300 m; distribution: known from one population from Paramo de Sumapaz in the Department of Meta, Colombia; conservation status: CR B2ab (ii, iii) AOO = 4 km2; etymology: dedicated to A. M. Cleef, who collected the type specimen.
Myrosmodes subnivalis Szlach., Mytnik and S. Nowak sp. nov. (Fig. 3)
This species is allied to Myrosmodes nubigenum Rchb.f., but the lip is thin, obtrullate in outline, without fleshy pads in sinus between the middle and lateral lobes, the lateral lip lobes thin and delicate.
Type: J. Aguirre C. and A.M. Cleef 889-Colombia, Santander, Paramo del Almorzadero, desde el paramo bajo hasta superparamo, alt. 3,900 m (17–19 Nov 1978), (COL! 411155 holotype).
Plant to 4.5 cm tall, enclothed in numerous, scarious, semitransparent bracts. Leaves 5–6, gathered in the basal rosette, petiolate; petiole 0.5 cm long; blade 2.5 × 0.8 cm, lanceolate to oblong-lanceolate, subobtuse to subacute. Inflorescence 2 cm long, densely many-flowered. Flowers small. Floral bracts 11 mm long, ligulate-ovate, rounded at apex, semitransparent. Ovary 6 mm long. Sepaline tube to 3 mm long. Dorsal sepal 3 × 1.2 mm, oblong-ovate, rounded at apex, 1-nerved. Petals 3 × 0.5 mm, linear to linear-lanceolate, falcate, acute, apical margin erose, 1-nerved. Lateral sepals 4.5 × 1.7 mm, lanceolate-ovate, somewhat sigmoid, acute, apical margin erose. Lip 4.5 mm long and wide, obtrullate in outline, thin, with 3 branching nerves, slightly thickened apically, ciliate along midnerve, with 2 subglobose, mamilliform thickenings at the base, apical half densely covered by long hairs resembling strings of beads, 3-lobed; the middle lobe 1.4 × 1 mm, oblong-ovate, obtuse; lateral lobes thin; no fleshy pads in sinus between lobes.
Ecology: terrestrial in paramo and superparamo, on wet rocks between lateral moraines, between glacier; altitude: 3,720–4,500 m; distribution: known from Ecuador and Colombia; conservation status: EN B2ab(ii, iii) AOO = 16 km2; etymology: collected at the highest elevation at which a Neotropical orchid has ever been reported (4,500 m.a.s.l.).
Representative specimens: Barclay and P. Juajibioy 7426-Colombia, Boyaca, Cordillera Oriental, Sierra Nevada del Cocuy, Alto Ritacuva, on high, wet rocks between lateral moraines, between tongue of glacier, alt. 4,500 m (11–29 Apr 1959), (COL!); S. Diaz P., H. Valencia Z. and R. Jaramillo M. 1759-Colombia, Risaralda, Mpio, de Pereira, Alrededores de la Laguna Otun, terrestre sobre cojines de Plantago rigida, alt. 4,000–4,300 m (3 Feb 1980), (COL!).
Other materials examined: Barclay and P. Juajibioy 9084-Ecuador, Napo-Pastaza, alrededores de La Cordillera de Los Llanganates, Chuchila Sacha o Ainchilibi, between grass clumps, lower slopes of Ainchilibi above cienga (Chihuila Sacha), alt. 3,720 m (25–29 Aug 1959), (COL!).
Myrosmodes reticulata Szlach., Mytnik and S. Nowak sp. nov. (Fig. 4)
This species is related to Myrosmodes nubigenum Rchb.f., but the lip is subquadrate in outline, with stiff, oblong, truncate middle lip lobe. Venation of the lip is strongly thickened, forming a very characteristic net.
Type: R. Jaramillo M., van der Hammen, A.M. Cleef and O. Rangel 5655-Colombia, Risaralda, Cordillera Central, Mpio, de Pereira, Parquet de los Navados, Nevado de Santa Isabel, vertiente WNW, superparamo bajo, morrena reciente, alt. 4,400–4,450 m (17 Jan 1980), (COL! 218517 holotype).
Plants to 10 cm tall, erect, enclothed by scarious, semitransparent bracts. Leaves 4–7, gathered in the basal rosette, petiolate; petiole 1–1.5 cm long; blade to 1.5 × 1.2 cm, ovate to broadly ovate, obtuse to subobtuse. Inflorescence 3 cm long, densely many-flowered. Flowers small. Floral bracts to 22 mm long, semitransparent, scarious. Ovary 3.5 mm long. Sepaline tube 1 mm long. Dorsal sepal 3.5 × 2.8 mm, ovate-triangular, subobtuse, concave, thin. Petals 3.5 × 0.8 mm, linear-lanceolate, obtuse to subobtuse, sigmoid, with some hairs on margins, 1-nerved. Lateral sepals 3.6 × 3 mm wide, obliquely oblong-ovate to elliptic, obtuse, concave, thin. Lip 4.2 mm long in total, concave, thin, subquadrate in outline, nerves branching and anastomosing, forming a prominent net, 3-lobed at apex; the middle lobe 1.2 mm long, stiff, oblong, truncate at apex; basal part 3 × 4 mm, truncate at apex, pubescent on apical margin and somewhat undulate. Gynostemium 3 mm long.
Ecology: terrestrial in superparamo; altitude: 4,400–4,450 m; distribution: known so far from one population from the Department of Risaralda, Colombia; conservation status: CR B2ab(ii, iii) AOO = 4 km2; etymology: in reference to very characteristic nervation net of the lip.
Myrosmodes has weak representation in herbarium specimens, so it can be assumed that this genus is rare in the environment. Additionally, orchids have specific biology, such as symbiosis with fungus needed for germination of seeds or tight adaption to pollinators; in some cases, self-pollination is possible, especially in harsh ecosystems such as paramo and puna, but this is unprofitable in terms of population genetic condition. Furthermore, human impacts have drastically increased over the last 20 years in both paramo and puna (Gondard 1988; De Koning and Veldkamp 1998). Activities such as cattle grazing and introduction of exotic species, often in cultivation and others related with human inhabitance, have had important and negative influence on vegetation composition and structure, ratio of ground biomass, hydrological behavior of the system, and chemical and physical properties of the soils (Hofstede et al. 1995; Ramsay and Oxley 1997; Poulenard et al. 2001, 2004; Farley et al. 2004; Cuesta and De Bievre 2008). Paramo is highly fragmented, which can be a barrier to propagation for plants living in this formation, and thus also for species of Myrosmodes. Additionally, only 43.4 % of all paramo in Andes is formally protected (Cuesta and De Bievre 2008). In our opinion, all these factors provide strong support for the conservation status proposed for the new species described herein (IUCN 2010).
The new species described here are restricted in distribution to the Andes in Colombia and Ecuador, part of the Tropical Andes biodiversity hotspot (Mittermeier et al. 2004). The Tropical Andes hotspot contains the richest and most diverse flora on Earth (Mittermeier et al. 2004). In addition, many Andean areas, such as parts of the Department of Meta in Colombia or Cordillera de Los Llanganates in Ecuador, are unexplored and their species diversity is still poorly known (Davis et al. 1997). The newly described species are known from four protected areas of IUCN category II: in Colombia from Sumapaz National Park (Myrosmodes cleefi), Los Nevados Natural National Park (M. reticulata and M. subnivalis), and Sierra Nevada del Cocuy Chita o Guican Natural National Park (M. subnivalis), and in Ecuador from environs of Llanganates National Park (M. subnivalis). These areas embrace largely paramo formation, with Sierra Nevada del Cocuy representing the highest diversity and proportion of endemism in the paramos of the East Cordillera. In the superparamo of this region there are about 20 endemic species, while in the superparamo of Sumapaz there are only 3 endemic species. The main threats to the paramo in these locations are intensive sheep and cattle grazing, occasionally with burning, wood cutting, and mining development (Davis et al. 1997).
The three new species are endemic to tropical Andes in Colombia and Ecuador; furthermore, Myrosmodes cleefi and M. reticulata are known from single localities in Colombia. They occur in paramos, which are heavily impacted. Based on these facts, in our opinion M. cleefi and M. reticulate lie in the critically endangered IUCN category (CR B2ab) whereas M. subnivalis should be considered an endangered taxon (EN B2ab).
References
Álvarez-Molina A, Cameron KM (2009) Molecular phylogenetics of Prescottiinae s.l and their close allies (Orchidaceae, Cranichideae) inferred from plastid and nuclear ribosomal DNA sequences. Amer J Bot 96:1020–1040
Anthelme F, Buendia B, Mazoyer C, Dangles O (2011) Unexpected mechanisms sustain the stress gradient hypothesis in a tropical alpine environment. J Veg Sci. doi:10.1111/j.1654-1103.2011.01333.x
Berry PE, Calvo RN (1991) Pollinator limitation and position dependent fruit set in the high Andean orchid Myrosmodes cochleare (Orchidaceae). Pl Syst Evol 174:93–101
Cleef AM (1981) The vegetation of the Páramos of the Colombian Cordillera Oriental. Dissertationes Botanicae 61. J. Cramer, Vaduz
Cuatrecasas J (1968) Páramo vegetation and its life forms. Colloquium Geographicum 9:163–186
Cuesta FC, De Bievre B (2008) Northern Andes Grasslands. In: Michelson A (ed) Temperate grasslands of South America. TGCI, China, pp 3–11
Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J, Hamilton A (eds) (1997) Centres of plant diversity: a guide and strategy for their conservation. IUCN Publications Unit, England
De Koning A, Veldkamp LO (1998) Land use in Ecuador: a statistical analysis at different aggregation levels. Agric Ecosyst Environ 70:231–247
Dressler RL (1990) The Spiranthoideae: grade or subfamily? Lindleyana 5:110–116
Dressler RL (1993) Phylogeny and classification of the orchid family. Dioscorides, USA
Farley KA, Kelly EF, Hofstede RGM (2004) Soil organic carbon and water retention after conversion of grasslands to pine plantations in the Ecuadorian Andes. Ecosystems 7:729–739
Garay LA (1978) Orchidaceae. Cypripedioideae Orchidoideae Neottioideae. In: Harling G, Sparre B (eds) Flora of Ecuador, 225(1). University of Goteborg, Stockholm, Sweden
García E, Beck SG (2006) Punas. In: Moraes RM, Øllgaard B, Kvist LP, Borchsenius F, Balslev H (eds) Botánica económica de los Andes centrales. Universidad Mayor de San Andrés, La Paz, pp 51–76
Gondard P (1988) Land use in the Andean region of Ecuador: from inventory to analysis. Land Use Policy 5:341–348
Halloy S, Beck SG, Ledezma JC (2008) Central and high Andean grasslands. In: Michelson A (ed) Temperate grasslands of South America. TGCI, China, pp 3–11
Harling G (1979) The vegetation types of Ecuador—a brief survey. In: Larsen K, Holm-Nielsen LB (eds) Tropical botany. Academic, New York, pp 165–174
Hofstede RGM, Chilito EJ, Sandoval EM (1995) Vegetative structure, microclimate, and leaf growth of a páramo tussock grass species, in undisturbed, burned and grazed conditions. Vegetatio 119:53–65
IUCN (2010) Guidelines for Using the IUCN Red List Categories and Criteria: Version 8.1. IUCN Species Survival Commission. IUCN, UK
Jørgensen PM, Ulloa CU (1994) Seed plants of the high Andes of Ecuador—a checklist. AAU Rep 34:1–443
Josse C, Cuesta F, Navarro G, Barrena V, Cabrera E, Chacón-Moreno E, Ferreira W, Peralvo M, Saito J, Tovar A (2008) Ecosistemas de los Andes del Norte y Centrales. Bolivia, Colombia, Ecuador, Peru y Venezuela. CAN, Programa Regional ECOBONA, CONDESAN-Proyecto Páramo Andino, Programa BioAndes, EcoCiencia, NatureServe, LTA-UNALM, IAvH, ICAE-ULA, CDCUNALM, RUMBOL SRL. Lima
Luteyn JL (1992) Páramos: why study them? In: Balslev H, Luteyn JL (eds) Páramo: an Andean ecosystem under human influence. Academic, London, pp 1–14
Mittermeier RA, Robles Gil P, Hoffman M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, da Fonseca GAB (2004) Hotspots revisited. CEMEX, Mexico, pp 73–82
Poulenard J, Podwojewski P, Janeau JL, Collinet J (2001) Runoff and soil erosion under rainfall simulation of andisols from the Ecuadorian páramo: effect of tillage and burning. Catena 45:185–207
Poulenard J, Michel JC, Bartoli F, Portal M, Podwojewski P (2004) Water repellency of volcanic ash soils from Ecuadorian páramo: effect of water content and characteristics of hydrophobic organic matter. Eur J Soil Sci 55:487–496
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (2003) Genera Orchidacearum:Orchidoideae (part 2), Vanilloideae. Oxford University Press, USA, pp 40–42
Ramsay PM (1992) The Páramo Vegetation of Ecuador: The Community Ecology Dynamics and Productivity of Tropical Grasslands in the Andes, Ph.D., thesis, University of Wales, Bangor
Ramsay PM, Oxley ERB (1997) The growth form composition of plant communities in the Ecuadorian páramos. Plant Ecol 131:73–192
Reichenbach HG (1854) Altensteinia, Aa and Myrosmodes. Xenia Orchid 1:17–19
Reichenbach HG (1878) Orchideae Mandonianae. Xenia Orchid 3:17–19
Rundel PW (1994) Tropical alpine climates. In: Rundel PW, Smith AP, Meinzer FC (eds) Tropical alpine environments: Plant, form and function 21–59. Cambridge University Press, USA
Schlechter R (1912) Die Orchideen Gattungen Altensteinia HBK, Aa Rchb.f. und Myrosmodes Rchb.f. Repert Spec Nov Regni Veg 11:147–150
Schweinfurth C (1958) Orchids of Peru. Fieldiana Bot 30(1):1–260
Szlachetko DL (1995) Systema orchidalium. Fragm Flor Geobot 3:1–152
Szlachetko DL, Rutkowski P (2000) Gynostemia Orchidalium:Volume 1. Apostasiaceae, Cypripediaceae, Orchidaceae (Thelymitroideae to Vanilloideae). Acta Bot Fen 169:234
Trujillo D, Vargas CA (2011) New species of Aa and new combinations in Myrosmodes (Orchidaceae: Cranichidinae) from Bolivia and Peru. Lankesteriana 11(1):1–8
Vargas C (1997) Phylogenetic analysis of Cranichideae and Prescottiinae (Orchidaceae) with some taxonomic changes in Prescottiinae, M.S., thesis, University of Missouri-St. Luis, St. Louis Missouri, USA
Acknowledgments
We would like to thank Dr. Carlos Parra, the curator of COL, for his support during the visits to the herbarium. We also wish to thank an anonymous reviewer for critical comments. Special thanks go to Anna Król, who made the drawings of the species.
Conflict of interest
The paper is part of a project supported by a grant from the Polish Ministry of Science and Higher Education (3930/PO1/2007/33).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mytnik-Ejsmont, J., Szlachetko, D.L. & Nowak, S. Three new species of Myrosmodes (Orchidaceae, Spiranthoideae) from Colombia and Ecuador. Plant Syst Evol 298, 1909–1916 (2012). https://doi.org/10.1007/s00606-012-0690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-012-0690-9