Abstract
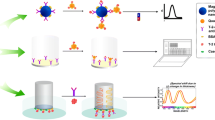
The development of a bioluminescent immunosensor is reported for the determination of zearalenone (ZEA) based on a peptide mimetic identified by phage display. The peptide mimetic GW, with a peptide sequence GWWGPYGEIELL, was used to create recombinant fusion proteins with the bioluminescent Gaussia luciferase (GLuc) that were directly used as tracers for toxin detection in a competitive immunoassay without the need for secondary antibodies or further labeling. The bioluminescent sensor, based on protein G–coupled magnetic beads for antibody immobilization, enabled determination of ZEA with a detection limit of 4.2 ng mL−1 (corresponding to 420 μg kg−1 in food samples) and an IC50 value of 11.0 ng mL−1. The sensor performance was evaluated in spiked maize and wheat samples, with recoveries ranging from 87 to 106% (RSD < 20%, n = 3). Finally, the developed method was applied to the analysis of a naturally contaminated reference matrix material and good agreement with the reported concentrations was obtained.
Graphical abstract





Similar content being viewed by others
References
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16:497–516. https://doi.org/10.1128/CMR.16.3.497-516.2003
DeVries JW, Trucksess MW, Jackson LS (2002) Mycotoxins and food safety. Springer, New York, NY, USA
Council for Agricultural Science and Technology, CAST (2003) Mycotoxins: risks in plant, animal, and human systems. Council for agricultural science and technology. Ames, Iowa
Bräse S, Encinas A, Keck J, Nising CF (2009) Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev 109:3903–3990. https://doi.org/10.1021/cr050001f
European Food Safety Authority, EFSA Panel on Contaminants in the Food Chain (2017) Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J 15:2197. https://doi.org/10.2903/j.efsa.2017.4851
Goyal S, Ramawat KG, Mérillon JM (2016) Different shades of fungal metabolites: an overview. In: Merillon J-M, Ramawat KG (eds) Fungal Metabolites. Springer, Cham
European Commission (2006) Commission regulation (EC) no 1881/2006. Off J Eur Union L364:5–24
European Commission (2007) Commission regulation (EC) no 1126/2007. Off J Eur Union L255:14–17
U.S. Food and Drug Administration (FDA). www.fda.gov/food/guidanceregulation/. Accessed 28 July 2020
European Commission (2006) Commission recommendation (EC) no 576/2006. Off J Eur Union L229:7–10
De Saeger S (2011) Determining mycotoxins and mycotoxigenic fungi in food and feed. Woodhead Publishing, Cambridge
Turner NW, Subrahmanyam S, Piletsky SA (2009) Analytical methods for determination of mycotoxins: a review. Anal Chim Acta 632:168–180. https://doi.org/10.1016/j.aca.2008.11.010
De Saeger S, Sibanda L, Van Peteghem C (2003) Analysis of zearalenone and α-zearalenol in animal feed using high-performance liquid chromatography. Anal Chim Acta 487:137–143. https://doi.org/10.1016/S0003-2670(03)00555-5
Drzymala SS, Weiz S, Heinze J, Marten S, Prinz C, Zimathies A, Garbe LA, Koch M (2015) Automated solid-phase extraction coupled online with HPLC-FLD for the quantification of zearalenone in edible oil. Anal Bioanal Chem 407:3489–3497. https://doi.org/10.1007/s00216-015-8541-5
Romera D, Mateo EM, Mateo-Castro R, Gómez JV, Gimeno-Adelantado JV, Jiménez M (2018) Determination of multiple mycotoxins in feedstuffs by combined use of UPLC–MS/MS and UPLC–QTOF–MS. Food Chem 267:140–148. https://doi.org/10.1016/j.foodchem.2017.11.040
Hidalgo-Ruiz JL, Romero-González R, Martínez Vidal JL, Garrido Frenich A (2019) A rapid method for the determination of mycotoxins in edible vegetable oils by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem 288:22–28. https://doi.org/10.1016/j.foodchem.2019.03.003
Köppen R, Koch M, Siegel D, Merkel S, Maul R, Nehls I (2010) Determination of mycotoxins in foods: current state of analytical methods and limitations. Appl Microbiol Biotechnol 86:1595–1612. https://doi.org/10.1007/s00253-010-2535-1
Berthiller F, Brera C, Crews C, Iha MH, Krska R, Lattanzio VMT, MacDonald S, Malone RJ, Maragos C, Solfrizzo M, Stroka J, Whitaker TB (2016) Developments in mycotoxin analysis: an update for 2014–2015. World Mycotoxin J 9:5–30. https://doi.org/10.3920/WMJ2015.1998
Turner NW, Bramhmbhatt H, Szabo-Vezse M, Poma A, Coker R, Piletsky SA (2015) Analytical methods for determination of mycotoxins: an update (2009–2014). Anal Chim Acta 901:12–33. https://doi.org/10.1016/j.aca.2015.10.013
Hervás M, López MA, Escarpa A (2011) Integrated electrokinetic magnetic bead-based electrochemical immunoassay on microfluidic chips for reliable control of permitted levels of zearalenone in infant foods. Analyst 136:2131–2138. https://doi.org/10.1039/C1AN15081B
Xu S, Zhang G, Fang B, Xiong Q, Duan H, Lai W (2019) Lateral flow immunoassay based on polydopamine-coated gold nanoparticles for the sensitive detection of zearalenone in maize. ACS Appl Mater Interfaces 11:31283–31290. https://doi.org/10.1021/acsami.9b08789
Yang M, Cui M, Wang W, Yang Y, Chang J, Hao J, Wang H (2020) Background-free upconversion-encoded microspheres for mycotoxin detection based on a rapid visualization method. Anal Bioanal Chem 412:81–91. https://doi.org/10.1007/s00216-019-02206-1
Azri FA, Eissa S, Zourob M, Chinnappan R, Sukor R, Yusof NA, Raston NHA, Alhoshani A, Jinap S (2020) Electrochemical determination of zearalenone using a label-free competitive aptasensor. Microchim Acta 187:266. https://doi.org/10.1007/s00604-020-4218-7
Xing K-Y, Peng J, Shan S, Liu DF, Huang YN, Lai WH (2020) Green enzyme-linked immunosorbent assay based on the single-stranded binding protein-assisted aptamer for the detection of mycotoxin. Anal Chem 92:8422–8426. https://doi.org/10.1021/acs.analchem.0c01073
Peltomaa R, Benito-Peña E, Moreno-Bondi MC (2018) Bioinspired recognition elements for mycotoxin sensors. Anal Bioanal Chem 410:747–771. https://doi.org/10.1007/s00216-017-0701-3
Chauhan R, Singh J, Sachdev T, Basu T, Malhotra BD (2016) Recent advances in mycotoxins detection. Biosens Bioelectron 81:532–545. https://doi.org/10.1016/j.bios.2016.03.004
Wild D (2013) The immunoassay handbook: theory and applications of ligand binding, ELISA and related techniques, 4th edn. Elsevier, Amsterdam
Zhao F, Wang H, Han X, Yang Z (2016) Development and comparative study of chemosynthesized antigen and mimotope-based immunoassays for class-specific analysis of O,O-dimethyl organophosphorus pesticides. Sci Rep 6. https://doi.org/10.1038/srep37640
Gazarian T, Selisko B, Hérion P, Gazarian K (2000) Isolation and structure–functional characterization of phage display library-derived mimotopes of noxiustoxin, a neurotoxin of the scorpion Centruroides noxius Hoffmann. Mol Immunol 37:755–766. https://doi.org/10.1016/S0161-5890(00)00091-2
Liu J, Chisti MM, Zeng X (2017) General signal amplification strategy for nonfaradic impedimetric sensing: trastuzumab detection employing a peptide immunosensor. Anal Chem 89:4013–4020. https://doi.org/10.1021/acs.analchem.6b04570
Wang Y, Wang H, Li P, Zhang Q, Kim HJ, Gee SJ, Hammock BD (2013) Phage-displayed peptide that mimics aflatoxins and its application in immunoassay. J Agric Food Chem 61:2426–2433. https://doi.org/10.1021/jf4004048
He Z, He Q, Xu Y, Li YP, Liu X, Chen B, Lei D, Sun CH (2013) Ochratoxin a mimotope from second-generation peptide library and its application in immunoassay. Anal Chem 85:10304–10311. https://doi.org/10.1021/ac402127t
He Q, Xu Y, Zhang C, Li YP, Huang ZB (2014) Phage-borne peptidomimetics as immunochemical reagent in dot-immunoassay for mycotoxin zearalenone. Food Control 39:56–61. https://doi.org/10.1016/j.foodcont.2013.10.019
Barbas CFI, Burton DR, Scott JK, Silverman GJ (2001) Phage display: a laboratory manual. Cold Spring Harbor laboratory press. Cold Spring Harbor, NY, USA
Peltomaa R, López-Perolio I, Benito-Peña E, Barderas R, Moreno-Bondi MC (2016) Application of bacteriophages in sensor development. Anal Bioanal Chem 408:1805–1828. https://doi.org/10.1007/s00216-015-9087-2
Peltomaa R, Benito-Peña E, Barderas R, Moreno-Bondi MC (2019) Phage display in the quest for new selective recognition elements for biosensors. ACS Omega 4:11569–11580. https://doi.org/10.1021/acsomega.9b01206
Smartt AE, Ripp S (2011) Bacteriophage reporter technology for sensing and detecting microbial targets. Anal Bioanal Chem 400:991–1007. https://doi.org/10.1007/s00216-010-4561-3
Peltomaa R, Benito-Peña E, Barderas R, Sauer U, González Andrade M, Moreno-Bondi MC (2017) Microarray-based immunoassay with synthetic mimotopes for the detection of fumonisin B1. Anal Chem 89:6216–6223. https://doi.org/10.1021/acs.analchem.7b01178
Zou X, Chen C, Huang X, Chen X, Wang L, Xiong Y (2016) Phage-free peptide ELISA for ochratoxin a detection based on biotinylated mimotope as a competing antigen. Talanta 146:394–400. https://doi.org/10.1016/j.talanta.2015.08.049
Liu R, Yu Z, He Q, Xu Y (2007) An immunoassay for ochratoxin a without the mycotoxin. Food Control 18:872–877. https://doi.org/10.1016/j.foodcont.2006.05.002
Peltomaa R, Agudo-Maestro I, Más V, Barderas R, Benito-Peña E, Moreno-Bondi MC (2019) Development and comparison of mimotope-based immunoassays for the analysis of fumonisin B1. Anal Bioanal Chem 411:6801–6811. https://doi.org/10.1007/s00216-019-02068-7
Peltomaa R, Amaro-Torres F, Carrasco S, Orellana G, Benito-Peña E, Moreno-Bondi MC (2018) Homogeneous quenching immunoassay for fumonisin B 1 based on gold nanoparticles and an epitope-mimicking yellow fluorescent protein. ACS Nano 12:11333–11342. https://doi.org/10.1021/acsnano.8b06094
Xu Y, Chen B, He Q, Qiu YL, Liu X, He ZY, Xiong ZP (2014) New approach for development of sensitive and environmentally friendly immunoassay for mycotoxin fumonisin B1 based on using peptide–MBP fusion protein as substitute for coating antigen. Anal Chem 86:8433–8440. https://doi.org/10.1021/ac502037w
Xu Y, He Z, He Q, Qiu Y, Chen B, Chen J, Liu X (2014) Use of cloneable peptide–MBP fusion protein as a mimetic coating antigen in the standardized immunoassay for mycotoxin ochratoxin a. J Agric Food Chem 62:8830–8836. https://doi.org/10.1021/jf5028922
Ding Y, Hua X, Du M et al (2018) Recombinant, fluorescent, peptidomimetic tracer for immunodetection of imidaclothiz. Anal Chem 90:13996–14002. https://doi.org/10.1021/acs.analchem.8b03685
Ding Y, Hua X, Chen H, Liu F, González-Sapien G, Wang M (2018) Recombinant peptidomimetic-nano luciferase tracers for sensitive single-step immunodetection of small molecules. Anal Chem 90:2230–2237. https://doi.org/10.1021/acs.analchem.7b04601
Tannous BA (2009) Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc 4:582–591
Moutsiopoulou A, Hunt E, Broyles D, Pereira CA, Woodward K, Dikici E, Kaifer A, Daunert S, Deo SK (2017) Bioorthogonal protein conjugation: application to the development of a highly sensitive bioluminescent immunoassay for the detection of interferon-γ. Bioconjug Chem 28:1749–1757. https://doi.org/10.1021/acs.bioconjchem.7b00220
New England Biolabs (NEB) Ph.D. Phage Display Libraries - Instruction Manual. www. neb.com/products/e8110-phd-12-phage-display-peptide-library-kit. Accessed 28 July 2020
Hunt EA, Moutsiopoulou A, Ioannou S, Ahern K, Woodward K, Dikici E, Daunert S, Deo SK (2016) Truncated variants of Gaussia luciferase with tyrosine linker for site-specific bioconjugate applications. Sci Rep 6:26814. https://doi.org/10.1038/srep26814
Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom RCM 20:2649–2659. https://doi.org/10.1002/rcm.2640
Marco M-P, Gee S, Hammock BD (1995) Immunochemical techniques for environmental analysis I. Immunosensors. Trends Anal Chem 14:341–350. https://doi.org/10.1016/0165-9936(95)97062-6
Zearalenone ELISA. Demeditec Diagn. GmbH. https://www.demeditec.com/en/products/zearalenone-elisa-dezeae03. Accessed 28 July 2020
Zearalenone ELISA Kit, Biovision. www.biovision.com/zearalenone-zen-elisa-kit.html. Accessed 28 July 2020
AgraQuant® Zearalenone ELISA test, Romer Labs. www.romerlabs.com/shop/romer_us_it/agraquant-r-zearalenone-elisa-test. Accessed 28 July 2020
Chen Y, Zhang S, Hong Z, Lin Y, Dai H (2019) A mimotope peptide-based dual-signal readout competitive enzyme-linked immunoassay for non-toxic detection of zearalenone. J Mater Chem B 7:6972–6980. https://doi.org/10.1039/C9TB01167F
Fang D, Zhang S, Dai H, Li X, Hong Z, Lin Y (2019) Electrochemiluminescent competitive immunoassay for zearalenone based on the use of a mimotope peptide, Ru(II)(bpy)
3-loaded NiFe2O4 nanotubes and TiO2 mesocrystals. Microchim Acta 186:608. https://doi.org/10.1007/s00604-019-3714-0Maragos CM, Kim EK (2006) Cross-reactivity of six zearalenone antibodies in a hand-held fluorescence polarisation immunoassay. In: Njapau H, Trujillo S, van Egmond H, Park D (eds) Mycotoxins and phycotoxins: advances in determination, toxicology and exposure management. Wageningen Academic Publishers
Acknowledgments
Belen Patiño from the Department of Biology (Universidad Complutense de Madrid) is gratefully acknowledged for providing the blank maize samples analyzed in this work.
Funding
This study was supported by the Spanish Ministry of Science, Innovation and Universities (RTI2018–096410-B-C21). R.P. acknowledges UCM for a research grant. S. Daunert and S. Deo would like to thank the National Institutes of Health, NIGMS (R01GM114321, R01GM127706). This work was also supported by the National Science Foundation grant (CHE-1506740, CBET-1841419) to S. Daunert and S. Deo. S. Daunert is grateful to the Miller School of Medicine of the University of Miami for the Lucille P. Markey Chair in Biochemistry and Molecular Biology.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors, and all authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2126 kb)
Rights and permissions
About this article
Cite this article
Peltomaa, R., Fikacek, S., Benito-Peña, E. et al. Bioluminescent detection of zearalenone using recombinant peptidomimetic Gaussia luciferase fusion protein. Microchim Acta 187, 547 (2020). https://doi.org/10.1007/s00604-020-04538-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04538-7