Abstract
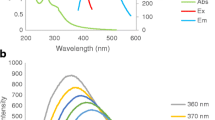
A highly selective fluorescent probe for Hg2+ is reported. It consists of nitrogen doped graphene quantum dots (NGQDs) that are nearly spherical in shape, have an average diameter of 2.7 nm and excitation-independent emission. The blue fluorescence of the NGQDs (with maximum excitation/emission at 378/447 nm) is quenched by Hg2+ due to both dynamic and static quenching. The probe has a wide detection range (2.5 μM – 800 μM) and a limit of detection of 2.5 μM. The dynamic and static quenching constants are 417 M−1 and 63500 M−1, respectively. The probe was used to quantfy Hg2+ in spiked real water samples with satisfactory results.

Graphical abstract






Similar content being viewed by others
References
Yu NX, Peng HL, Xiong H, Wu XQ, Wang XY, Li YB, Chen LX (2015) Graphene quantum dots combined with copper (II) ions as a fluorescent probe for turn-on detection of sulfide ions. Microchim Acta 182(13–14):2139–2146
Wang G, Guo QL, Chen D, Liu ZD, Zheng XH, Xu AL, Yang SW, Ding GQ (2018) Facile and highly effective synthesis of controllable lattice sulfur-doped graphene quantum dots via hydrothermal treatment of durian. ACS Appl Mater Interfaces 10(6):5750–5759
Shen C, Ge SY, Pang YY, Xi FN, Liu JY, Dong XP, Chen P (2017) Facile and scalable preparation of highly luminescent N, S co-doped graphene quantum dots and their application for parallel detection of multiple metal ions. J Mater Chem B 5(32):6593–6600
Wu X, Tian F, Wang WX, Chen J, Wu M, Zhao JXJ (2013) Fabrication of highly fluorescent graphene quantum dots using L-glutamic acid for in vitro/in vivo imaging and sensing. J Mater Chem C 1(31):4676–4684
Wang L, Zheng J, Li YH, Yang S, Liu CH, Xiao Y, Li JS, Cao Z, Yang RH (2014) AgNP-DNA@GQDs hybrid: new approach for sensitive detection of H2O2 and glucose via simultaneous AgNP etching and DNA cleavage. Anal Chem 86(24):12348–12354
Fan L, Hu Y, Wang X, Zhang L, Li F, Han D, Li Z, Zhang Q, Wang Z, Niu L (2012) Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta 101:192–197
Achadu OJ, Revaprasadu N (2018) Microwave-assisted synthesis of thymine-functionalized graphitic carbon nitride quantum dots as a fluorescent nanoprobe for mercury (II). Microchim Acta 185(10):461
Liu YF, Tang XS, Deng M, Zhu T, Bai YZ, Qu DR, Huang XB, Qiu F (2018) One-step aqueous synthesis of highly luminescent hydrophilic AgInZnS quantum dots. J Lumin 202:71–76
Liu YF, Deng M, Zhu T, Tang XS, Han S, Huang W, Shi YL, Liu AP (2017) The synthesis of water-dispersible zinc doped AgInS2 quantum dots and their application in Cu2+ detection. J Lumin 192:547–554
Ananthanarayanan A, Wang XW, Routh P, Sana B, Lim S, Kim DH, Lim KH, Li J, Chen P (2014) Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv Funct Mater 24(20):3021–3026
Fang BY, Li C, Song YY, Tan F, Cao YC, Zhao YD (2018) Nitrogen-doped graphene quantum dot for direct fluorescence detection of Al3+ in aqueous media and living cells. Biosens Bioelectron 100:41–48
Shamsipur M, Safavi A, Mohammadpour Z, Ahmadi R (2016) Highly selective aggregation assay for visual detection of mercury ion based on competitive binding of sulfur-doped carbon nanodots to gold nanoparticles and mercury ions. Microchim Acta 183(7):2327–2335
Koneswaran M, Narayanaswamy R (2009) L-cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sensors Actuat B–Chem 139(1):104–109
Wang L, Li WT, Wu B, Li Z, Wang SL, Liu Y, Pan DY, Wu MH (2016) Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem Eng J 300:75–82
Zhang M, Bai LL, Shang WH, Xie WJ, Ma H, Fu YY, Fang DC, Sun H, Fan LZ, Han M (2012) Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J Mater Chem 22(15):7461–7467
Yan ZY, Qu XC, Niu QQ, Tian CQ, Fan CJ, Ye BF (2016) A green synthesis of highly fluorescent nitrogen-doped graphene quantum dots for the highly sensitive and selective detection of mercury (II) ions and biothiols. Anal Methods 8(7):1565–1571
Baruah U, Chowdhury D (2016) Functionalized graphene oxide quantum dot–PVA hydrogel: a colorimetric sensor for Fe2+, Co2+ and Cu2+ ions. Nanotechnology 27(14):145501
Liu YF, Deng M, Tang XS, Zhu T, Zang ZG, Zeng XF, Han S (2016) Luminescent AIZS-GO nanocomposites as fluorescent probe for detecting copper(II) ion. Sensor Actuat B–Chem 233:25–30
Li L, Li L, Wang C, Liu K, Zhu R, Qiang H, Lin Y (2015) Synthesis of nitrogen-doped and amino acid-functionalized graphene quantum dots from glycine, and their application to the fluorometric determination of ferric ion. Microchim Acta 182(3–4):763–770
Liu YF, Zhu T, Deng M, Tang XS, Han S, Liu AP, Bai YZ, Qu DR, Huang XB, Qiu F (2018) Selective and sensitive detection of copper (II) based on fluorescent zinc-doped AgInS2 quantum dots. J Lumin 201:182–188
Liu YF, Tang XS, Zhu T, Deng M, Ikechukwu IP, Yin GL, Bai YZ, Qu DR, Huang XB, Qiu F (2018) All–inorganic CsPbBr3 perovskite quantum dots as photoluminescent probe for ultrasensitive Cu2+ detection. J Mater Chem C 6:4793–4799
Li DJ, Nie F, Tang TT, Tian KL (2018) Determination of ferric ion via its effect on the enhancement of the chemiluminescece of the permanganate-sulfite system by nitrogen-doped graphene quantum dots. Microchim Acta 185(9):431
Gu D, Shang S, Yu Q, Shen J (2016) Green synthesis of nitrogen-doped carbon dots from lotus root for Hg (II) ions detection and cell imaging. Appl Surf Sci 390:38–42
Su D, Wang M, Liu Q, Qu Z, Su X (2018) A novel fluorescence strategy for mercury ion and trypsin activity assay based on nitrogen-doped graphene quantum dots. New J Chem 42(20):17083–17090
Alvand M, Shemirani F (2017) A Fe3O4@SiO2@graphene quantum dot core-shell structured nanomaterial as a fluorescent probe and for magnetic removal of mercury (II) ion. Microchim Acta 184(6):1621–1629
Tang WJ, Wang Y, Wang PP, Di JW, Yang JP, Wu Y (2016) Synthesis of strongly fluorescent carbon quantum dots modified with polyamidoamine and a triethoxysilane as quenchable fluorescent probes for mercury(II). Microchim Acta 183(9):2571–2578
Peng D, Zhang L, Liang R-P, Qiu J-D (2018) Rapid detection of mercury ions based on nitrogen-doped graphene quantum dots accelerating formation of manganese porphyrin. ACS Sensors 3(5):1040–1047
Li L, Yu B, You T (2015) Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (II) ions. Biosens Bioelectron 74:263–269
Wang B, Zhuo S, Chen L, Zhang Y (2014) Fluorescent graphene quantum dot nanoprobes for the sensitive and selective detection of mercury ions. Spectrochim Acta Part A 131:384–387
Tabaraki R, Sadeghinejad N (2018) Microwave assisted synthesis of doped carbon dots and their application as green and simple turn off–on fluorescent sensor for mercury (II) and iodide in environmental samples. Ecotox Environ Safe 153:101–106
Li Z, Wang Y, Ni YN, Kokot S (2015) A rapid and label-free dual detection of Hg (II) and cysteine with the use of fluorescence switching of graphene quantum dots. Sensors Actuators B Chem 207:490–497
Barman S, Sadhukhan M (2012) Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J Mater Chem 22(41):21832–21837
Fan LJ, Zhang Y, Murphy CB, Angell SE, Parker MFL, Flynn BR, Jones WE (2009) Fluorescent conjugated polymer molecular wire chemosensors for transition metal ion recognition and signaling. Coord Chem Rev 253(3):410–422
Zu F, Yan F, Bai Z, Xu J, Wang Y, Huang Y, Zhou X (2017) The quenching of the fluorescence of carbon dots: a review on mechanisms and applications. Microchim Acta 184(7):1899–1914
Gan T-T, Zhang Y-J, Zhao N-J, Xiao X, Yin G-F, Yu S-H, Wang H-B, Duan J-B, Shi C-Y, Liu W-Q (2012) Hydrothermal synthetic mercaptopropionic acid stabled CdTe quantum dots as fluorescent probes for detection of Ag+. Spectrochim Acta A Mol Biomol Spectrosc 99:627–668
Qu Z, Na W, Nie Y, Su X (2018) A novel fluorimetric sensing strategy for highly sensitive detection of phytic acid and hydrogen peroxide. Anal Chim Acta 1039:74–81
Xia YS, Zhu CQ (2008) Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 75(1):215–221
Acknowledgements
This work was supported by Science Foundation of China (61635004, 61705023, 61705024, 11574161, 61405023), Key Research and Development Project of Ministry of Science and Technology (2016YFC0801200), Chongqing Postdoctoral Program for Innovative Talents (CQBX201703), Postdoctoral Science Foundation of Chongqing (Xm2017047), Natural Science Foundation of Chongqing (cstc2018jcyjAX0644), Science and Technology on Plasma Physics Laboratory (6142A0403050817), and National Science Fund for Distinguished Young Scholars (61825501).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have on competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 322 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Tang, X., Deng, M. et al. Nitrogen doped graphene quantum dots as a fluorescent probe for mercury(II) ions. Microchim Acta 186, 140 (2019). https://doi.org/10.1007/s00604-019-3249-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3249-4