Abstract
The authors describe a flow cytometric immunoassay for aflatoxin B1 (AFB1). It has three distinct features: (a) Magnetic microspheres encoded with upconverting nanocrystals (UCNMMs) are used as fluorescent labels. These have the advantage of non-overlapping spectra and lacking crosstalk between the encoding signal and reporter signal via the low-energy near-infrared (NIR) light excitation; (b) phycoerythrin-labeled secondary antibodies are used to amplify the reporter signal; (c) The use of magnetic nanoparticles facilitates the rapid separation and specific purification of the analyte (AFB1). This assay has a detection limit of 9 pg·mL−1 and a broad working range for AFB1, requires a 50 μL sample only, and can be completed within 2 h with good accuracy and high reproducibility. It is perceived that such multifluorescent UCNMMs, whose color depends on the kind of dopants (Yb, Er, Tm, Mn) in the NaYF4 host lattice, represent a promising tool for the analysis of mycotoxins and other analytes.

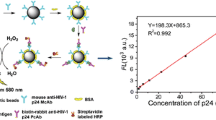
Schematic of the UCNMM-based indirective competitive immunoassay for AFB1 using the flow cytometric analysis (FCA) technology. The UCNMMs are prepared by doping the upconversion nanocrystals and magnetic nanoparticles inside the mesoporous polystyrene microspheres as the self-healing encapsulation strategy.





Similar content being viewed by others
References
Creppy EE (2002) Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol Lett 127(1–3):19–28. doi:10.1016/S0378-4274(01)00479-9
Hussein HS, Brasel JM (2001) Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 167(2):101–134. doi:10.1016/S0300-483X(01)00471-1
Beizaei A, O’ Kane SL, Kamkar A, Misaghi A, Henehan G, Cahill DJ (2015) Highly sensitive toxin microarray assay for aflatoxin B1 detection in cereals. Food Control 57:210–215. doi:10.1016/j.foodcont.2015.03.039
Min W-K, Kweon D-H, Park K, Park Y-C, Seo J-H (2011) Characterisation of monoclonal antibody against aflatoxin B1 produced in hybridoma 2C12 and its single-chain variable fragment expressed in recombinant Escherichia coli. Food Chem 126(3):1316–1323. doi:10.1016/j.foodchem.2010.11.088
Guchi E (2015) Implication of aflatoxin contamination in agricultural products. American Journal of Food and Nutrition 3(1):12–20. doi:10.12691/ajfn-3-1-3
Rahmani A, Jinap S, Soleimany F (2009) Qualitative and quantitative analysis of mycotoxins. Compr Rev Food Sci Food Saf 8(3):202–251. doi:10.1111/j.1541-4337.2009.00079.x
Li Z, Yu Y, Li Z, Wu T (2015) A review of biosensing techniques for detection of trace carcinogen contamination in food products. Anal Bioanal Chem 407(10):2711–2726
Rahmani A, Jinap S, Soleimany F (2010) Validation of the procedure for the simultaneous determination of aflatoxins ochratoxin a and zearalenone in cereals using HPLC-FLD. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27(12):1683–1693
Zhang Z, Hu X, Zhang Q, Li P (2016) Determination for multiple mycotoxins in agricultural products using HPLC-MS/MS via a multiple antibody immunoaffinity column. J Chromatogr B Anal Technol Biomed Life Sci 1021:145–152
He T, Wang Y, Li P, Zhang Q, Lei J, Zhang Z, Ding X, Zhou H, Zhang W (2014) Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Anal Chem 86(17):8873–8880
Deng G, Xu K, Sun Y, Chen Y, Zheng T, Li J (2013) High sensitive immunoassay for multiplex mycotoxin detection with photonic crystal microsphere suspension array. Anal Chem 85(5):2833–2840
Chauhan R, Singh J, Sachdev T, Basu T, Malhotra BD (2016) Recent advances in mycotoxins detection. Biosens Bioelectron 81:532–545
Wang Y, Liu N, Ning B, Liu M, Lv Z, Sun Z, Peng Y, Chen C, Li J, Gao Z (2012) Simultaneous and rapid detection of six different mycotoxins using an immunochip. Biosens Bioelectron 34(1):44–50
Malhotra BD, Srivastava S, Ali MA, Singh C (2014) Nanomaterial-based biosensors for food toxin detection. Appl Biochem Biotechnol 174(3):880–896. doi:10.1007/s12010-014-0993-0
Ko J, Lee C, Choo J (2015) Highly sensitive SERS-based immunoassay of aflatoxin B1 using silica-encapsulated hollow gold nanoparticles. J Hazard Mater 285:11–17
Lv X, Li Y, Cao W, Yan T, Li Y, Du B, Wei Q (2014) A label-free electrochemiluminescence immunosensor based on silver nanoparticle hybridized mesoporous carbon for the detection of aflatoxin B 1. Sensors Actuators B Chem 202(4):53–59
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2014) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Mikrochimica Acta 182:571–578
Lee J, Jeon CH, Ahn SJ, Ha TH (2014) Highly stable colorimetric aptamer sensors for detection of ochratoxin a through optimizing the sequence with the covalent conjugation of hemin. Analyst 139(7):1622–1627. doi:10.1039/C3AN01639K
Yue S, Jie X, Wei L, Bin C, Dou WD, Yi Y, Qingxia L, Jianlin L, Tiesong Z (2014) Simultaneous detection of ochratoxin a and fumonisin B1 in cereal samples using an aptamer-photonic crystal encoded suspension array. Anal Chem 86(23):11797–11802
Bonetta L (2005) Flow cytometry smaller and better. Nat Methods 2(10):785–795
Ren W, Liu H, Yang W, Fan Y, Yang L, Wang Y, Liu C, Li Z (2013) A cytometric bead assay for sensitive DNA detection based on enzyme-free signal amplification of hybridization chain reaction. Biosens Bioelectron 49C(22):380–386
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A (2004) Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol 110(3):252–266. doi:10.1016/j.clim.2003.11.017
Vignali DAA (2000) Multiplexed particle-based flow cytometric assays. J Immunol Methods 243(1–2):243–255. doi:10.1016/S0022-1759(00)00238-6
Wang H-Q, Liu T-C, Cao Y-C, Huang Z-L, Wang J-H, Li X-Q, Zhao Y-D (2006) A flow cytometric assay technology based on quantum dots-encoded beads. Anal Chim Acta 580(1):18–23. doi:10.1016/j.aca.2006.07.048
Sathe TR, Agrawal A, Nie S (2006) Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: dual-function Microcarriers for optical encoding and magnetic separation. Anal Chem 78(16):5627–5632. doi:10.1021/ac0610309
Zhang Y, Dong C, Su L, Wang H, Gong X, Wang H, Liu J, Chang J (2016) Multifunctional microspheres encoded with upconverting nanocrystals and magnetic nanoparticles for rapid separation and immunoassays. ACS Appl Mater Interfaces 8(1):745–753. doi:10.1021/acsami.5b09913
Gorris HH, Wolfbeis OS (2013) Photon-upconverting nanoparticles for optical encoding and multiplexing of cells, biomolecules, and microspheres. Angew Chem 52(13):3584
Wang M, Mi C-C, Wang W-X, Liu C-H, Wu Y-F, Xu Z-R, Mao C-B, Xu S-K (2009) Immunolabeling and NIR-excited fluorescent imaging of HeLa cells by using NaYF4:Yb,Er upconversion nanoparticles. ACS Nano 3(6):1580–1586. doi:10.1021/nn900491j
Wu S, Duan N, Wang Z, Wang H (2011) Aptamer-functionalized magnetic nanoparticle-based bioassay for the detection of ochratoxin a using upconversion nanoparticles as labels. Analyst 136(11):2306–2314. doi:10.1039/C0AN00735H
Zhang F, Shi Q, Zhang Y, Shi Y, Ding K, Zhao D, Stucky GD (2011) Fluorescence upconversion Microbarcodes for multiplexed biological detection: nucleic acid encoding. Adv Mater 23(33):3775–3779. doi:10.1002/adma.201101868
Wang H, Liu Z, Wang S, Dong C, Gong X, Zhao P, Chang J (2014) MC540 and upconverting Nanocrystal Coloaded polymeric liposome for near-infrared light-triggered photodynamic therapy and cell fluorescent imaging. ACS Appl Mater Interfaces 6(5):3219–3225. doi:10.1021/am500097f
Tang D, Zhong Z, Niessner R, Knopp D (2009) Multifunctional magnetic bead-based electrochemical immunoassay for the detection of aflatoxin B1 in food. Analyst 134(8):1554–1560. doi:10.1039/B902401H
Song E, Han W, Li J, Jiang Y, Cheng D, Song Y, Zhang P, Tan W (2014) Magnetic-encoded fluorescent multifunctional Nanospheres for simultaneous multicomponent analysis. Anal Chem 86(19):9434–9442. doi:10.1021/ac5031286
Guo Y, Tian J, Liang C, Zhu G, Gui W (2013) Multiplex bead-array competitive immunoassay for simultaneous detection of three pesticides in vegetables. Microchim Acta 180(5):387–395
Wang Y, Ning B, Peng Y, Bai J, Liu M, Fan X, Sun Z, Lv Z, Zhou C, Gao Z (2013) Application of suspension array for simultaneous detection of four different mycotoxins in corn and peanut. Biosens Bioelectron 41:391–396. doi:10.1016/j.bios.2012.08.057
Guo X, Wen F, Zheng N, Luo Q, Wang H, Wang H, Li S, Wang J (2014) Development of an ultrasensitive aptasensor for the detection of aflatoxin B 1. Biosens Bioelectron 56C(1):340–344
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182(3–4):571–578
Acknowledgements
The authors gratefully acknowledge National Natural Science Foundation of China (31401578, 51373117, 51303126, 51573128 and 81402575), Tianjin Natural Science Foundation (13JCZDJC33200 and 15JCQNJC03100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no competing financial interests.
Electronic supplementary material
ESM 1
(DOCX 0.97 mb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Liao, Z., Liu, Y. et al. Flow cytometric immunoassay for aflatoxin B1 using magnetic microspheres encoded with upconverting fluorescent nanocrystals. Microchim Acta 184, 1471–1479 (2017). https://doi.org/10.1007/s00604-017-2116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2116-4