Abstract
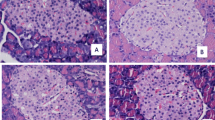
Exposure to aluminium is associated with altered glycaemia, but this effect in relation to glucose tolerance and pancreatic islet pathology requires validation. The study was aimed at assessing the glycaemic changes, glucose tolerance and islet morphology of albino rats during oral aluminium chloride exposure. Twenty male albino rats weighing 189–270 g were randomly divided into two equal groups. The control group was sham-treated with normal saline and the treated group was administered aluminium chloride solution (100 g/L) by gavage at 12.5% of LD50 (50 mg/kg) daily for 28 days. Fasting blood glucose (FBG) and postprandial blood glucose (PBG) concentrations after glucose gavage at 1 g/kg were estimated and the pancreatic islet tissues were examined for histopathological changes. The treatment caused significant (p < 0.05) time-dependent (r = 0.97) increases in FBG. Hyperglycaemic effect was estimated to be 28-day glycaemic increase of 61% and aggregate glycaemic increases of 44% and 53% between and within groups, respectively. Oral glucose tolerance was impaired judging by changes in PBG which indicated reduction of both intestinal absorption and clearance of blood glucose in treated group by 37% and 17%, respectively. Coagulative necrosis and 31% reduction (p < 0.05) in cell count of the pancreatic islet tissue were associated with the deranged circulatory glucose homeostasis in the treated group. In conclusion, aluminium loading seems to affect insulin production and action and may have a contributory role in diabetogenesis.






Similar content being viewed by others
References
Afridi HI, Talpur FN, Kazi TG, Brabazon D (2015) Effect of trace and toxic elements of different brands of cigarettes on the essential elemental status of Irish referent and diabetic mellitus consumers. Trace Elem Res 167(2):209–224
Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH (2009) Heavy metals, islet function and diabetes development. Islets 1(3):169–176
EFSA (2008) Safety of aluminium from dietary intake. EFSA J 754:1–34
Feng W, Cui X, Liu B, Liu C, Xiao Y, Lu W (2015) Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One 10:e0123742
Flores CR, Puga MP, Wrobel K, Sevilla MEG, Wrobel K (2011) Trace elements status in diabetes mellitus type 2: possible role of the interaction between molybdenum and copper in the progress of typical complications. Diabetes Res Clin Pract 91:333–341
Gonzalez MA, Alvarez ML, Pisani GB (2007) Involvement of oxidative stress in the impairment in biliary secretory function induced by intraperitoneal administration of aluminum to rats. Biol Trace Elem Res 116(1):329–348
Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D et al (2011) Na (+)-D-SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61:187–196
Igbokwe IO, Shamaki LS, Hamza H, Gidado A (1999) Fasting hyperglycaemia with oral glucose tolerance in acute Trypanosoma congolense infection of rats. Vet Parasitol 81:167–171
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Johnson VJ, Kim SH, Sharma RP (2005) Aluminium-maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol Sci 83(2):329–339
Kellett G, Helliwell P (2009) The diffuse component of intestinal absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 350:155–162
Konda VR, Eerike M, Chary RP, Arunachalam R, Yeddula VR, Meti V, Devi TS (2017) Effect of aluminum chloride on blood glucose level and lipid profile in normal, diabetic and treated diabetic rats. Indian J Pharm 49:357–365
Krasovskii GN, Vasukovich LY, Chariev OG (1979) Experimental study of biological effects of lead and aluminum following oral administration. Environ Health Perspect 30:47–51
Mailloux RJ, Hamel R, Appanna VD (2006) Aluminum toxicity elicits a dysfunctional TCA cycle and succinate accumulation in hepatocytes. J Biochem Mol Toxicol 20:198–208
Mirhashemi SM, Aarabi MH (2011) To study various concentrations of magnesium and aluminium on amylin hormone conformation. Pak J Biol Sci 14(11):653–657
Mirhashemi SM, Shahabaddin M-E (2011) Evaluation of aluminium, manganese, copper and selenium effects on human islets amyloid polypeptide hormone aggregation. Pak J Biol Sci 14(4):288–292
Moshtaghie A, Ani M (1991) Interferance of aluminium with carbohydrate metabolism in male rats: a model study of dialysis patients. J Sci Islamic Republic of Iran 2(1,2):1–4
Obukhova T, Budkar’ LN, Tereshina LG, Karpova EA (2015) [dissociation of disorders of carbohydrate and lipid metabolism in aluminum industry workers according to medical examination data] in Russian. Gig Sanit 94(2):67–69
Reusche E, Lindner B, Arnholbt H (1994) Widespread aluminium deposition in extracerebral organ systems of patients with dialysis-associated encephalopathy. Virchows Arch 424:105–112
Rheney CC, Kirk KK (2000) Performance of three blood glucose meters. Ann Pharmacother 34(3):317–321
Russel C, Palmer JE, Boston RC, Wilkins PA (2007) Agreement between point-of-care glucometry, blood gas and laboratory-based measurement of glucose in an equine neonatal intensive care unit. J Vet Emerg Crit Care 17(3):236–242
Serdar MA, Bakir F, Hasimi A, Celik T, Akin O, Kenar L, Aykut O, Yildirimkaya M (2009) Trace and toxic element patterns in nonsmoker patients with noninsulin-dependent diabetes mellitus, impaired glucose tolerance, and fasting glucose. Int J Diabetes Dev C 29:35–40
van der Voet GB (1992) Interstinal absorption of aluminium. CIBA Found Symp 169:109–117
Vignal C, Desreumaux P, Body-Malapel M (2016) Gut: an underestimated target organ for aluminium. Morphologie 100(329):75–84
Wei H, Wang MD, Meng LX (2012) Laurel West aluminum industrial base residents trace element content in serum and its associated abnormal glucose metabolism (J). Mod Prev Med 4:065
Wei X, Wei H, Yang D, Li D, Yang X, He M, Lin E, Wu B (2018) Effect of aluminum exposure on glucose metabolism and its mechanism in rats. Biol Trace Elem Res 186:450–456. First online 28 March 2018. https://doi.org/10.1007/s12011-018-1318-x
Xu ZX, Fox S, Melethel L, Winderg I, Badr M (1990) Mechanism of aluminium-induced inhibition of hepatic glycolysis. J Pharmacol Exp Ther 254:301–305
Xu Z-X, Zhang Q, Ma G-L, Chen C-H, He Y-M, Xu L-H, Zhang Y, Zhou G-R, Li Z-H, Yang H-J, Zhou P (2016, 2016) Influence of aluminium and EGCCG on fibrillation and aggregation of human islet amyloid polypeptide. J Diabetes Res:1867059, 14 pages. https://doi.org/10.1155/2016/1867059
Zhou Y, Harris WR, Yokel RA (2008) The influence of citrate, maltolate and fluoride on the gastrointestinal absorption of aluminum at a drinking water-relevant concentration: a 26Al and 14C study. J Inorg Biochem 102:798–808
Acknowledgements
Technical assistance was provided by Bitrus Wampana and Tijani Aji Goni.
Funding
Financial assistance was granted by the Council of the University of Maiduguri through a study fellowship to Ephraim Igwenagu.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical compliance
The research was approved by the School of Postgraduate Studies Board of the University of Maiduguri, Maiduguri, Nigeria, and complied with institutional, national and international standards for research on laboratory animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Igwenagu, E., Igbokwe, I.O. & Egbe-Nwiyi, T.N. Fasting hyperglycaemia, glucose intolerance and pancreatic islet necrosis in albino rats associated with subchronic oral aluminium chloride exposure. Comp Clin Pathol 29, 75–81 (2020). https://doi.org/10.1007/s00580-019-03028-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-03028-4