Abstract
Background
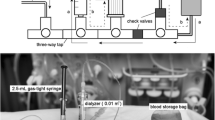
For continuous renal replacement therapy in small infants, due to the large extracorporeal volume involved, blood priming can be necessary to prevent hypotension and hemodilution. Because packed red blood cells (RBCs) have high levels of potassium and citrate, closed-circuit dialysis is often performed. We assessed the metrics of closed-circuit dialysis and serial citrate concentration changes.
Methods
We performed dialysis of closed circuits primed with expired human packed RBC solution and 5% albumin. Blood and dialysate flow rates were 70 and 33.3 mL/min, respectively. The extracorporeal volume was 70 mL. We measured pH, electrolytes, and citrate in the closed circuit every 3 min for 15 min. We also assessed the adequacy of closed-circuit dialysis using the formula: [dialysate flow rate (mL/min) × time of dialysis (min)]/extracorporeal volume (mL) and we assessed the correlation between citrate and ionized calcium concentrations.
Results
To reach normal concentrations of sodium, potassium, and chloride, 2.4 times as much dialysate fluid as extracorporeal volume was needed. In contrast, for ionized calcium, bicarbonate, and citrate, 3.8 times as much dialysate fluid as extracorporeal volume was required. By simple linear regression analysis, the concentration of citrate was significantly correlated with that of ionized calcium.
Conclusions
For closed-circuit dialysis using an RBC solution, the formula [dialysate flow rate (mL/min) × time of dialysis (min)]/extracorporeal volume (mL) would be a better parameter to estimate efficacy, compared with other metrics. Additionally, the citrate concentration can be readily estimated from the ionized calcium concentration during closed-circuit dialysis.



Similar content being viewed by others
References
Warady BA, Bunchman T (2000) Dialysis therapy for children with acute renal failure: survey results. Pediatr Nephrol 15:11–13
Sethi SK, Bunchman T, Raina R, Kher V (2014) Unique considerations in renal replacement therapy in children: core curriculum 2014. Am J Kidney Dis 63:329–345
Sousa CN, Gama M, Andrade M, Faria MS, Pereira E (2008) Haemodialysis for children under the age of two years. J Ren Care 34:9–13
Bunchman TE, Brophy PD, Goldstein SL (2008) Technical considerations for renal replacement therapy in children. Semin Nephrol 28:488–492
Sutherland SM, Alexander SR (2012) Continuous renal replacement therapy in children. Pediatr Nephrol 27:2007–2016
Dzik WH, Kirkley SA (1988) Citrate toxicity during massive blood transfusion. Transfus Med Rev 2:76–94
Garzotto F, Zanella M, Ronco C (2014) The evolution of pediatric continuous renal replacement therapy. Nephron Clin Pract 127:172–175
Pasko DA, Mottes TA, Mueller BA (2003) Pre dialysis of blood prime in continuous hemodialysis normalizes pH and electrolytes. Pediatr Nephrol 18:1177–1183
Hackbarth RM, Eding D, Gianoli Smith C, Koch A, Sanfilippo DJ, Bunchman TE (2005) Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid-base homeostasis prior to patient connection. Pediatr Nephrol 20:1328–1333
Raina R, Vijayaraghavan P, Kapur G, Sethi SK, Krishnappa V, Kumar D, Bunchman TE, Bolen SD, Chand D (2018) Hemodialysis in neonates and infants: a systematic review. Semin Dial 31:289–299
Oudemans-van Straaten HM, Ostermann M (2012) Bench-to-bedside review: citrate for continuous renal replacement therapy, from science to practice. Crit Care 16:249
Journois D, Israel-Biet D, Pouard P, Rolland B, Silvester W, Vouhé P, Safran D (1996) High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology 85:965–976
Wang S, Palanzo D, Ündar A (2012) Current ultrafiltration techniques before, during and after pediatric cardiopulmonary bypass procedures. Perfusion 27:438–446
Vraets A, Lin Y, Callum JL (2011) Transfusion-associated hyperkalemia. Transfus Med Rev 25:184–196
Schneider AG, Journois D, Rimmelé T (2017) Complications of regional citrate anticoagulation: accumulation or overload? Crit Care 21:281
Deep A, Stewart CE, Dhawan A, Douiri A (2016) Effect of continuous renal replacement therapy on outcome in pediatric acute liver failure. Crit Care Med 44:1910–1919
Schultheiß C, Saugel B, Phillip V, Thies P, Noe S, Mayr U, Haller B, Einwächter H, Schmid RM, Huber W (2012) Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: a prospective observational study. Crit Care 16:R162
Rodriguez K, Srivaths PR, Tal L, Watson MN, Riley AA, Himes RW, Desai MS, Braun MC, Akcan Arikan A (2017) Regional citrate anticoagulation for continuous renal replacement therapy in pediatric patients with liver failure. PLoS One 12:e0182134
Reddi BAJ (2013) Why is saline so acidic (and does it really matter?). Int J Med Sci 10:747–750
Acknowledgments
The hemofilters (EXCELFRO AEF-03; Asahi Kasei Medical Co., Ltd., Tokyo, Japan) were provided to D.S. by Asahi Kasei Medical Co., Ltd., Tokyo, Japan.
Funding
This work was supported by a Grant for Co-medical Staff Research to D.S. from the Japanese Society for Dialysis Therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The hemofilters (EXCELFRO AEF-03; Asahi Kasei Medical Co., Ltd., Tokyo, Japan) were provided to D.S. by Asahi Kasei Medical Co., Ltd., Tokyo, Japan. Other authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Ethical Committee of St. Luke’s International Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 75.0 kb)
Rights and permissions
About this article
Cite this article
Saito, D., Fujimaru, T., Inoue, Y. et al. Serial measurement of electrolyte and citrate concentrations in blood-primed continuous hemodialysis circuits during closed-circuit dialysis. Pediatr Nephrol 35, 127–133 (2020). https://doi.org/10.1007/s00467-019-04318-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04318-3