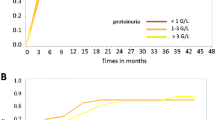
Abstract
Background
Henoch-Schönlein purpura (HSP) is the most common vasculitis in childhood and traditionally considered as a self-limiting disease. However, renal involvement can unfavorably determine long-term prognosis. The reported regimens to treat HSP nephritis (HSPN) are diverse, indicating that the most effective treatment remains controversial.
Methods
This retrospective, single-center study involved 18 patients presenting with HSPN and nephrotic-range proteinuria. We aimed to investigate the efficacy and safety of mycophenolate mofetil (MMF) and identify a cut-off level for estimated mycophenolic acid area under the curve (eMPA-AUC0-12h) values, which can predict complete remission with high sensitivity.
Results
Despite prior insufficient therapeutic response to corticosteroids, 89% of patients showed a significant decrease in proteinuria after 1 month of MMF treatment. None of them relapsed during treatment; however, two children relapsed after discontinuation. Based on results of a receiver operating characteristic (ROC) analysis, an eMPA-AUC0–12h >56.4 mg*h/l was a predictor for complete remission within 3 months (80% sensitivity, 83.3% specificity, p = 0.035). During MMF administration, we encountered no adverse event requiring discontinuation of treatment.
Conclusion
Our study demonstrates that MMF is a safe and potentially effective secondary treatment option for children with HSPN to achieve and maintain long-term remission without serious side effects. To achieve complete remission within 3 months, resolve severe inflammatory glomerular lesions, and avoid progression to chronic kidney disease, we propose timely diagnosis and early initiation of MMF with an eMPA-AUC0–12h value of 56.4 mg*h/l.



Similar content being viewed by others
Abbreviations
- ACEi:
-
Angiotensin-converting enzyme inhibitors
- ARB:
-
Angiotensin II receptor blockers
- BSA:
-
Body surface area
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- GTP:
-
Guanosine-5′-triphosphate
- HSP:
-
Henoch-Schönlein purpura
- HSPN:
-
Henoch-Schönlein purpura nephritis
- IgACI:
-
Immunoglobulin A circulating immune complex
- IMPDH:
-
Inosine-5′-monophosphate dehydrogenase
- IS therapy:
-
Immunosuppressive therapy
- MMF:
-
Mycophenolate mofetil
- MP:
-
Methylprednisolone
- MPA-C0 :
-
Pre-dose level of mycophenolic acid
- eMPA-AUC0-12h :
-
Estimated mycophenolic acid–area under the concentration versus time curve
- NA:
-
Not applicable
- PU:
-
Proteinuria
- RAAS:
-
Renin–angiotensin–aldosterone system
- ROC:
-
Receiver operating characteristic
- TDM:
-
Therapeutic drug monitoring
- UTI:
-
Urinary tract infection
References
Aalberse J, Dolman K, Ramnath G, Pereira RR, Davin JC (2007) Henoch Schonlein purpura in children: an epidemiological study among Dutch paediatricians on incidence and diagnostic criteria. Ann Rheum Dis 66:1648–1650
Davin JC, Coppo R (2014) Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol 10:563–573
Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR (2002) Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 360:1197–1202
Pohl M (2015) Henoch-Schönlein purpura nephritis. Pediatr Nephrol 30:245–252
Jauhola O, Ronkainen J, Koskimies O, Ala-Houhala M, Arikoski P, Hölttä T, Jahnukainen T, Rajantie J, Ormälä T, Turtinen J, Nuutinen M (2010) Renal manifestations of Henoch-Schonlein purpura in a 6-month prospective study of 223 children. Arch Dis Child 95:877–882
North American Pediatric Renal Trials and Collaborative Studies; Annual Transplant Report (2014). Available from: https://web.emmes.com/study/ped/annlrept/annualrept2014.pdf
KDIGO Work Group (2012) KDIGO clinical practice guideline for glomerulonephritis, Chapter 11: Henoch-Schönlein purpura nephritis. Kidney Int Suppl 2, 218–220. https://doi.org/10.1038/kisup.2012.24
Hahn D, Hodson EM, Willis NS, Craig JC (2015) Interventions for preventing and treating kidney disease in Henoch-Schönlein Purpura (HSP). Cochrane Database Syst Rev 7(8):CD005128
Weiss PF, Feinstein JA, Luan X, Burnham JM, Feudtner C (2007) Effects of corticosteroid on Henoch-Schönlein purpura: a systematic review. Pediatrics 120:1079–1087
Niaudet P, Habib R (1998) Methylprednisolone pulse therapy in the treatment of severe forms of Schönlein-Henoch purpura nephritis. Pediatr Nephrol 12:238–243
Li Volti S, Li Volti G (2003) Early treatment with oral immunosuppressants in severe proteinuric purpura nephritis. Pediatr Nephrol 18:1197–1198
Tanaka H, Suzuki K, Nakahata T, Ito E, Waga S (2003) Early treatment with oral immunosuppressants in severe proteinuric purpura nephritis. Pediatr Nephrol 18:347–350
Tarshish P, Bernstein J, Edelmann CM Jr (2004) Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol 19:51–56
Park JM, Won SC, Shin JI, Yim H, Pai KS (2011) Cyclosporin A therapy for Henoch-Schönlein nephritis with nephrotic-range proteinuria. Pediatr Nephrol 26:411–417
Jauhola O, Ronkainen J, Autio-Harmainen H, Koskimies O, Ala-Houhala M, Arikoski P, Hölttä T, Jahnukainen T, Rajantie J, Ormälä T, Nuutinen M (2011) Cyclosporine a vs. methylprednisolone for Henoch-Schönlein nephritis: a randomized trial. Pediatr Nephrol 26:2159–2166
Kawasaki Y, Suyama K, Hashimoto K, Hosoya M (2011) Methylprednisolone pulse plus mizoribine in children with Henoch-Schoenlein purpura nephritis. Clin Rheumatol 30(4):529–535
Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47:85–118
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42:1114–1120
Baudouin V, Alberti C, Lapeyraque AL, Bensman A, André JL, Broux F, Cailliez M, Decramer S, Niaudet P, Deschênes G, Jacqz-Aigrain E, Loirat C (2012) Mycophenolate mofetil for steroid-dependent nephrotic syndrome: a phase II Bayesian trial. Pediatr Nephrol 27:389–396
Gellermann J, Weber L, Pape L, Tönshoff B, Hoyer P, Querfeld U, Gesellschaft für Pädiatrische Nephrologie (GPN) (2013) Mycophenolate mofetil versus cyclosporin a in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol 24:1689–1697
Hackl Á, Cseprekál O, Gessner M, Liebau MC, Habbig S, Ehren R, Müller C, Taylan C, Dötsch J, Weber LT (2016) Mycophenolate Mofetil therapy in children with Idiopathic Nephrotic Syndrome: does therapeutic drug monitoring make a difference? Ther Drug Monit 38:274–279
Dede F, Onec B, Ayli D, Gonul II, Onec K (2008) Mycophenolate mofetil treatment of crescentic Henoch-Schönlein nephritis with IgA depositions. Scand J Urol Nephrol 42:178–180
Ren P, Han F, Chen L, Xu Y, Wang Y, Chen J (2012) The combination of mycophenolate mofetil with corticosteroids induces remission of Henoch-Schönlein purpura nephritis. Am J Nephrol 36:271–277
Han F, Chen LL, Ren PP, Le JY, Choong PJ, Wang HJ, Xu Y, Chen JH (2015) Mycophenolate mofetil plus prednisone for inducing remission of Henoch-Schönlein purpura nephritis: a retrospective study. J Zhejiang Univ Sci B 16:772–779
Du Y, Hou L, Zhao C, Han M, Wu Y (2012) Treatment of children with Henoch-Schönlein purpura nephritis with mycophenolate mofetil. Pediatr Nephrol 27:765–771
Nikibakhsh AA, Mahmoodzadeh H, Karamyyar M, Hejazi S, Noroozi M, Macooie AA (2014) Treatment of severe henoch-schonlein purpura nephritis with mycophenolate mofetil. Saudi J Kidney Dis Transpl 25:858–863
Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J (2017) IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int 91:1014–1021
Heaton JM, Turner DR, Cameron JS (1977) Localization of glomerular “deposits” in Henoch--Schönlein nephritis. Histopathology 1:93–104
Pohl M, Dittrich K, Ehrich JHH, Hoppe B, Kemper MJ, Klaus G, Schmitt CP, Hoyer PF (2013) Gesellschaft für Pädiatrische Nephrologie (GPN) (2013) Behandlung der Purpura-Schönlein-Henoch-Nephritis bei Kindern und Jugendlichen; Therapieempfehlungen der Gesellschaft für Pädiatrische Nephrologie (GPN). Monatsschr Kinderheilkd 161:543–553
Weber LT, Hoecker B, Armstrong VW, Oellerich M, Tönshoff B (2008) Long-term pharmacokinetics of mycophenolic acid in pediatric renal transplant recipients over 3 years posttransplant. Ther Drug Monit 30:570–575
Wakaki H, Ishikura K, Hataya H, Hamasaki Y, Sakai T, Yata N, Kaneko T, Honda M (2011) Henoch-Schönlein purpura nephritis with nephrotic state in children: predictors of poor outcomes. Pediatr Nephrol 26:921–925
Coppo R, Amore A (2004) New perspectives in treatment of glomerulonephritis. Pediatr Nephrol 19:256–265
Davin JC, Coppo R (2013) Pitfalls in recommending evidence-based guidelines for a protean disease like Henoch-Schönlein purpura nephritis. Pediatr Nephrol 28:1897–1903
Ronkainen J, Ala-Houhala M, Huttunen NP, Jahnukainen T, Koskimies O, Ormälä T, Nuutinen M (2003) Outcome of Henoch-Schoenlein nephritis with nephrotic-range proteinuria. Clin Nephrol 60:80–84
Xie L, Tan C, Fan J, Fu P, Tang Y, Tao Y, Qin W (2013) Mycophenolic acid reverses IgA1 aberrant glycosylation through up-regulating Cosmc expression in IgA nephropathy. Int Urol Nephrol 45:571–579
Hackl A, Ehren R, Weber LT (2016) Effect of mycophenolic acid in experimental, nontransplant glomerular diseases: new mechanisms beyond immune cells. Pediatr Nephrol 32:1315–1322
Wang W, Mo S, Chan L (1999) Mycophenolic acid inhibits PDGF-induced osteopontin expression in rat mesangial cells. Transplant Proc 31:1176–1177
Dubus I, Vendrely B, Christophe I, Labouyrie JP, Delmas Y, Bonnet J, Combe C (2002) Mycophenolic acid antagonizes the activation of cultured human mesangial cells. Kidney Int 62:857–867
Badid C, Vincent M, McGregor B, Melin M, Hadj-Aissa A, Veysseyre C, Hartmann DJ, Desmouliere A, Laville M (2000) Mycophenolate mofetil reduces myofibroblast infiltration and collagen III deposition in rat remnant kidney. Kidney Int 58:51–61
Takeda S, Takahashi M, Sado Y, Takeuchi K, Hakamata Y, Shimizu H, Kaneko T, Yamamoto H, Ito C, Ookawara S, Asano Y, Kusano E, Kobayashi E (2004) Prevention of glomerular crescent formation in glomerulonephritis by mycophenolate mofetil in rats. Nephrol Dial Transplant 19:2228–2236
Weber LT, Shipkova M, Armstrong VW, Wagner N, Schütz E, Mehls O, Zimmerhackl LB, Oellerich M, Tönshoff B (2002) The pharmacokinetic pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: a report of the german study group on mycophenolate mofetil therapy. J Am Soc Nephrol 13:759–768
Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain F, Lavaud S, Etienne I, Westeel PF, Hurault de Ligny B, Rostaing L, Thervet E, Szelag JC, Rérolle JP, Rousseau A, Touchard G, Marquet P (2007) Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant 7:2496–2503
Tellier S, Dallocchio A, Guigonis V, Saint-Marcoux F, Llanas B, Ichay L, Bandin F, Godron A, Morin D, Brochard K, Gandia P, Bouchet S, Marquet P, Decramer S, Harambat J (2016) Mycophenolic acid pharmacokinetics and relapse in children with steroid-dependent idiopathic Nephrotic syndrome. Clin J Am Soc Nephrol 11:1777–1782
Hale MD, Nicholls AJ, Bullingham RE, Hené R, Hoitsma A, Squifflet JP, Weimar W, Vanrenterghem Y, Van de Woude FJ, Verpooten GA (1998) The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 64:672–683
Oellerich M, Shipkova M, Schütz E, Wieland E, Weber L, Tönshoff B, Armstrong VW (2000) Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit 22:20–26
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hackl, A., Becker, J.U., Körner, L.M. et al. Mycophenolate mofetil following glucocorticoid treatment in Henoch-Schönlein purpura nephritis: the role of early initiation and therapeutic drug monitoring. Pediatr Nephrol 33, 619–629 (2018). https://doi.org/10.1007/s00467-017-3846-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3846-6