Abstract
Background
Children with severe Henoch–Schönlein purpura nephritis (HSPN) may progress to end-stage renal disease without appropriate treatment.
Objective
This study aimed to investigate the efficacy and safety of tacrolimus combined with glucocorticoids in the treatment of pediatric HSPN.
Methods
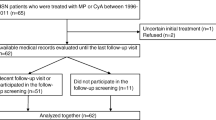
A total of 87 HSPN patients with urinary protein ≥ 0.75 g/24 h received standard of care, including angiotensin II receptor blockers/angiotensin-converting enzyme inhibitors and glucocorticoids. Patients were divided into three groups and additionally received tacrolimus (n = 30), cyclophosphamide (n = 31), or mycophenolate mofetil (MMF) (n = 26). We monitored outcome measures, including proteinuria, hematuria, and renal function and analyzed the efficacy and side effects in each group.
Results
At 2-month follow-up, the overall efficacy was 93.3%, 83.9%, and 61.5% for tacrolimus, cyclophosphamide, and MMF, respectively (P < 0.05). Urinary protein significantly decreased for all groups. Urinary red blood cell counts significantly decreased for patients treated with tacrolimus (P < 0.001) and cyclophosphamide (P < 0.05), whereas no significant decrease was seen for those receiving MMF (P = 0.09). Although urine β2-microglobulin significantly decreased following 2 months of treatment with all medications, efficacy was greater with tacrolimus than with cyclophosphamide and MMF (P < 0.001). Major adverse events were respiratory and urinary infections, with MMF having the highest infection rate. The cyclophosphamide group also experienced additional adverse events, including arrhythmia, hemorrhagic cystitis, leukocytosis, thrombocytopenia, and hyperglycemia.
Conclusions
These results indicate that tacrolimus is more effective at reducing proteinuria and hematuria and improving renal function, with relatively milder side effects, in the treatment of pediatric HSPN.
Clinical Trial Registration Number
ChiCTR2200055323, retrospectively registered on January 7, 2022.





Similar content being viewed by others
References
Gardner-Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360(9341):1197–202.
Leung AKC, Barankin B, Leong KF. Henoch-Schonlein Purpura in Children: An Updated Review. Curr Pediatr Rev. 2020 May 7.
Davin JC, Coppo R. Pitfalls in recommending evidence-based guidelines for a protean disease like Henoch-Schonlein purpura nephritis. Pediatr Nephrol. 2013;28(10):1897–903.
Suzuki H, Yasutake J, Makita Y, Tanbo Y, Yamasaki K, Sofue T, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93(3):700–5.
Nicoara O, Twombley K. Immunoglobulin A Nephropathy and Immunoglobulin A Vasculitis. Pediatr Clin North Am. 2019;66(1):101–10.
Sugiyama M, Wada Y, Kanazawa N, Tachibana S, Suzuki T, Matsumoto K, et al. A cross-sectional analysis of clinicopathologic similarities and differences between Henoch-Schönlein purpura nephritis and IgA nephropathy. PLoS ONE. 2020;15(4):e0232194.
Davin JC, Coppo R. Henoch-Schonlein purpura nephritis in children. Nat Rev Nephrol. 2014;10(10):563–73.
Ronkainen J, Nuutinen M, Koskimies O. The adult kidney 24 years after childhood Henoch-Schonlein purpura: a retrospective cohort study. Lancet. 2002;360(9334):666–70.
Wakaki H, Ishikura K, Hataya H, Hamasaki Y, Sakai T, Yata N, et al. Henoch-Schonlein purpura nephritis with nephrotic state in children: predictors of poor outcomes. Pediatr Nephrol. 2011;26(6):921–5.
Coppo R, Andrulli S, Amore A, Gianoglio B, Conti G, Peruzzi L, et al. Predictors of outcome in Henoch-Schönlein nephritis in children and adults. Am J Kidney Dis. 2006;47(6):993–1003.
KDIGO. Chapter 11: Henoch-Schönlein purpura nephritis. Kidney International Supplements. 2012;2(2):218–20.
Tanaka H, Suzuki K, Nakahata T, Ito E, Waga S. Early treatment with oral immunosuppressants in severe proteinuric purpura nephritis. Pediatr Nephrol. 2003;18(4):347–50.
Tarshish P, Bernstein J, Edelmann CM Jr. Henoch-Schonlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol. 2004;19(1):51–6.
Nephrology Subgroup of Chinese Pediatric Society CMA. Evidence-based guidelines for the diagnosis and treatment of pediatric Henoch-Schönlein purpura nephritis. Chin J Pediatr. 2017;55(9):647–51.
Lu Z, Song J, Mao J, Xia Y, Wang C. Evaluation of mycophenolate mofetil and low-dose steroid combined therapy in moderately severe Henoch-Schönlein purpura nephritis. Med Sci Monit. 2017;18(23):2333–9.
Yu Y, Chen J, Yin H, Deng Z, Xie Y, Yuan Q, et al. Efficacy of steroid and immunosuppressant combined therapy in Chinese patients with Henoch-Schönlein purpura nephritis: a retrospective study. Int Immunopharmacol. 2020;81:106229.
Song YH, Cai GY, Xiao YF, Wang YP, Yuan BS, Xia YY, et al. Efficacy and safety of calcineurin inhibitor treatment for IgA nephropathy: a meta-analysis. BMC Nephrol. 2017;18(1):61.
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis. 2010;69(5):798–806.
Toltl LJ, Arnold DM. Leaving nothing to chance: how to randomize a clinical trial. Transfusion. 2012;52(12):2513–5.
Jelusic M, Sestan M, Cimaz R, Ozen S. Different histological classifications for Henoch-Schonlein purpura nephritis: which one should be used? Pediatr Rheumatol Online J. 2019;17(1):10.
Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, et al. National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111(6 Pt 1):1416–21.
Davin JC, Ten Berge IJ, Weening JJ. What is the difference between IgA nephropathy and Henoch-Schönlein purpura nephritis? Kidney Int. 2001;59(3):823–34.
Hackl A, Becker JU, Korner LM, Ehren R, Habbig S, Nusken E, et al. Mycophenolate mofetil following glucocorticoid treatment in Henoch-Schonlein purpura nephritis: the role of early initiation and therapeutic drug monitoring. Pediatr Nephrol. 2018;33(4):619–29.
Zhang DF, Hao GX, Li CZ, Yang YJ, Liu FJ, Liu L, et al. Off-label use of tacrolimus in children with Henoch-Schonlein purpura nephritis: a pilot study. Arch Dis Child. 2018;103(8):772–5.
Huang X, Ma L, Ren P, Wang H, Chen L, Han H, et al. Updated Oxford classification and the international study of kidney disease in children classification: application in predicting outcome of Henoch-Schonlein purpura nephritis. Diagn Pathol. 2019;14(1):40.
Lim BJ, Shin JI, Choi SE, Rhim H, Lee JS, Kim PK, et al. The significance of tubulointerstitial lesions in childhood Henoch-Schonlein nephritis. Pediatr Nephrol. 2016;31(11):2087–93.
Mohkam M, Ghafari A. The role of urinary N-acetyl-beta-glucosaminidase in diagnosis of kidney diseases. J Pediatr Nephrol. 2015;3(3):84–91.
Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80(8):806–21.
Müller D, Greve D, Eggert P. Early tubular proteinuria and the development of nephritis in Henoch-Schönlein purpura. Pediatr Nephrol. 2000;15(1–2):85–9.
Chen X, Hou Y, Chen C, Jiang G. The predictive value of β2-microglobulin for steroid resistance in children with Henoch-Schönlein purpura nephritis. Int J Dermatol. 2020;59(10):e363–4.
Mise K, Hoshino J, Ueno T, Hazue R, Hasegawa J, Sekine A, et al. Prognostic Value of Tubulointerstitial Lesions, Urinary N-Acetyl-β-d-Glucosaminidase, and Urinary β2-Microglobulin in Patients with Type 2 Diabetes and Biopsy-Proven Diabetic Nephropathy. Clin J Am Soc Nephrol. 2016;11(4):593–601.
Assadi FK. Value of urinary excretion of microalbumin in predicting glomerular lesions in children with isolated microscopic hematuria. Pediatr Nephrol. 2005;20(8):1131–5.
Zhang Q, Shi SF, Zhu L, Lv JC, Liu LJ, Chen YQ, et al. Tacrolimus improves the proteinuria remission in patients with refractory IgA nephropathy. Am J Nephrol. 2012;35(4):312–20.
Rauch MC, San Martin A, Ojeda D, Quezada C, Salas M, Carcamo JG, et al. Tacrolimus causes a blockage of protein secretion which reinforces its immunosuppressive activity and also explains some of its toxic side-effects. Transpl Immunol. 2009;22(1–2):72–81.
Harrison CA, Bastan R, Peirce MJ, Munday MR, Peachell PT. Role of calcineurin in the regulation of human lung mast cell and basophil function by cyclosporine and FK506. Br J Pharmacol. 2007;150(4):509–18.
Eteghadi A, Pak F, Ahmadpoor P, Jamali S, Karimi M, Yekaninejad MS, et al. Th1, Th2, Th17 cell subsets in two different immunosuppressive protocols in renal allograft recipients (Sirolimus vs mycophenolate mofetil): a cohort study. Int Immunopharmacol. 2019;67:319–25.
Wakamatsu A, Fukusumi Y, Hasegawa E, Tomita M, Watanabe T, Narita I, et al. Role of calcineurin (CN) in kidney glomerular podocyte: CN inhibitor ameliorated proteinuria by inhibiting the redistribution of CN at the slit diaphragm. Physiological reports. 2016;4(6).
Wang L, Jirka G, Rosenberg PB, Buckley AF, Gomez JA, Fields TA, et al. Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest. 2015;125(5):1913–26.
Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, et al. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2010;21(10):1657–66.
Peng L, Ma J, Cui R, Chen X, Wei SY, Wei QJ, et al. The calcineurin inhibitor tacrolimus reduces proteinuria in membranous nephropathy accompanied by a decrease in angiopoietin-like-4. PLoS ONE. 2014;9(8):e106164.
Park SJ, Suh JS, Lee JH, Lee JW, Kim SH, Han KH, et al. Advances in our understanding of the pathogenesis of Henoch-Schönlein purpura and the implications for improving its diagnosis. Expert Rev Clin Immunol. 2013;9(12):1223–38.
Kidokoro K, Satoh M, Nagasu H, Sakuta T, Kuwabara A, Yorimitsu D, et al. Tacrolimus induces glomerular injury via endothelial dysfunction caused by reactive oxygen species and inflammatory change. Kidney Blood Press Res. 2012;35(6):549–57.
Kamdem LK, Streit F, Zanger UM, Brockmoller J, Oellerich M, Armstrong VW, et al. Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005;51(8):1374–81.
Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos Biol Fate Chem. 2006;34(5):836–47.
Alvarez-Elias AC, Garcia-Roca P, Velasquez-Jones L, Valverde S, Varela-Fascinetto G, Medeiros M. CYP3A5 genotype and time to reach tacrolimus therapeutic levels in renal transplant children. Transpl Proc. 2016;48(2):631–4.
Hooper DK, Fukuda T, Gardiner R, Logan B, Roy-Chaudhury A, Kirby CL, et al. Risk of tacrolimus toxicity in CYP3A5 nonexpressors treated with intravenous nicardipine after kidney transplantation. Transplantation. 2012;93(8):806–12.
Ghobadi E, Moloudizargari M, Asghari MH, Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin Drug Metab Toxicol. 2017;13(5):525–36.
Heineke MH, Ballering AV, Jamin A, Ben Mkaddem S, Monteiro RC, Van Egmond M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schonlein purpura). Autoimmun Rev. 2017;16(12):1246–53.
Acknowledgements
The authors thank the patients and their families for facilitating this work. This study was supported in part by startup funding from Nantong Maternity and Child Healthcare Hospital (to YY), the Lanzhou University Second Hospital Introduced Talent Research Project (ynyjrck-yzx2015-2-02, to YY), the Lanzhou University Second Hospital Cuiying Science and Technology Innovation Project (CY2017-MS16, to YY), and the Science and Technology Development Plan of Chengguan District, Lanzhou City, Gansu Province (2017KJGG0050, to YY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this study.
Ethics approval
The studies involving human participants were reviewed and approved by the Ethical Committee of Lanzhou University Second Hospital.
Consent to participate
Written informed consent to participate in this study was provided by the participants' parents or legal guardians.
Consent for publication
Written informed consent for publication was obtained from the participants' parents or legal guardians.
Availability of data and material (data transparency)
All the original data presented in the study are included in the article.
Code availability
Not applicable.
Author contributions
DW participated in the patient’s care, collected and analyzed the data, and prepared the manuscript. RM participated in the patient's care and collected the data. XW analyzed the data and made critical revision to the manuscript. YY participated in the patient's care, supervised the study, analyzed the data, and wrote the manuscript. All authors have read and approved the manuscript.
Rights and permissions
About this article
Cite this article
Wu, D., Ma, R., Wang, X. et al. Efficacy and Safety of Tacrolimus in the Treatment of Pediatric Henoch–Schönlein Purpura Nephritis. Pediatr Drugs 24, 389–401 (2022). https://doi.org/10.1007/s40272-022-00506-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00506-1