Abstract
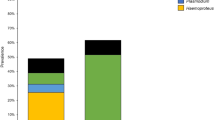
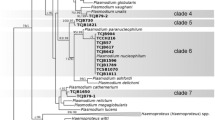
Haemosporidian blood parasites are transmitted to a wide range of avian hosts via blood-sucking dipteran vectors. Microscopy has revealed an impressive diversity of avian haemosporidia with more than 250 species described. Moreover, PCR and subsequent sequence analyses have suggested a much greater diversity of haemosporidia than morphological analyses alone. Given the importance of these parasites, very few studies have focused on the charismatic hummingbirds. To date, three Haemoproteus species (Haemoproteus archilochus, Haemoproteus trochili, and Haemoproteus witti) and one Leucocytozoon species (Leucocytozoon quynzae) have been described in blood samples taken from hummingbirds (Trochilidae). Unconfirmed Plasmodium lineages have also been detected in hummingbirds. Here, we report the detection of H. archilochus in two hummingbird species (Calypte anna and Archilochus alexandri) sampled in Northern California and perform a phylogenetic analysis of mitochondrial cytochrome b (cyt b) gene lineages. A total of 261 hummingbirds (157 C. anna, 104 A. alexandri) were sampled and screened for blood parasites using PCR and microscopy techniques. Combining both methods, 4 (2.55%) haemosporidian infections were detected in C. anna and 18 (17.31%) haemosporidian infections were detected in A. alexandri. Molecular analyses revealed four distinct H. archilocus cyt b lineages, which clustered as a monophyletic clade. No species of Plasmodium or Leucocytozoon were detected in this study, raising the possibility of specific vector associations with hummingbirds. These results provide resources for future studies of haemosporidian prevalence, diversity, and pathogenicity in California hummingbird populations.


Similar content being viewed by others
References
Aguirre AA, Tabor GM (2008) Global factors driving emerging infectious diseases. Ann N Y Acad Sci 1149:1–3. doi:10.1196/annals.1428.052
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215 (3):403–410
Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S (2015) Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347(6220):436–438. doi:10.1126/science.1261121
Baltosser WH, Russell SM (2000) Black-chinned hummingbird (Archilochus alexandri). In: Poole A, Gill F (eds) The birds of North America. No 495. The Birds of North America Inc, Philadelphia
Burton R (2001) The World of the Hummingbird. Firefly Books. Ontario, Canada
Buzato S, Sazima M, Sazima I (2000) Hummingbird-pollinated floras at three Atlantic Forest sites. Biotropica 32:824–841. doi:10.1111/j.1744-7429.2000.tb00621.x
Campbell TW, Dein FJ (1984) Avian hematology. The basics. Vet Clin N Am-Small 14(2):223–248
Carlson JS, Giannitti F, Valkiūnas G, Tell LA, Snipes J, Wright S (2016) A method to preserve low parasitaemia Plasmodium-infected avian blood for host and vector infectivity assays. Malar J 15:154
Coatney RG, West E (1938) Some blood parasites from Nebraska birds II. Am Midl Nat 19:601–612
Dusek RJ, Hall JS, Nashold SW, TeSlaa JL, Ip HS (2011) Evaluation of Nobuto filter paper strips for the detection of Avian Influenza virus antibody in waterfowl. Avian Diseases 55 (4):674–676
Earlé RA, Huchzermeyer FW, Bennett GF, Brossy JJ (1993) Babesia peircei sp. nov. from the jackass penguin. S Afr J Zool 28:88–90
Fallon S, Bermingham E, Ricklefs RE (2003) Island and taxon effects in parasitism revisited: avian malaria in the lesser Antilles. Evolution 57:606–615
Godoy LA, Tell LA, Ernest HB (2014) Hummingbird health: pathogens and disease conditions in the family Trochilidae. J Ornithol 155(1):1–12. doi:10.1007/s10336-013-0990-z
González AD, Lotta IA, García LF, Moncada LI, Matta NE (2015) Avian haemosporidians from Neotropical highlands: evidence from morphological and molecular data. Parasitol Int 64(4):48–59. doi:10.1016/j.parint.2015.01.007
Greiner EC, Bennett GF, White EM, Coombs RF (1975) Distribution of the avian hematozoa of North America. Can J Zool 53:1762–1787
Harrigan RJ, Sedano R, Chasar AC, Chaves JA, Nguyen JT, Whitaker A, Smith TB (2014) New host and lineage diversity of avian haemosporidia in the Northern Andes. Evol Appl 7:799–811. doi:10.1111/eva.12176
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802. doi:10.1645/GE-184R1
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649
Martínez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, García-Fraile S, Belda EJ (2010) The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 23:663–665
Marzal A, De Lope F, Navarro C, Møller AP (2005) Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia 142:541–545
Matta NE, Pacheco MA, Escalante AA, Valkiūnas G, Ayerbe-Quiñones F, Acevedo-Cendales LD (2014) Description of Leucocytozoon quynzae sp. nov. (Haemosporida, Leucocytozoidae) from hummingbirds, with remarks on distribution and possible vectors of leucocytozoids in South America. Parasitol Res 113:2991. doi:10.1007/s00436-014-3961-2
McGuire JA, Witt CC, Remsen JV, Corl A, Rabosky DL, Altshuler DL, Dudley R (2014) Molecular phylogenetics and the diversification of hummingbirds. Curr Bio 24:910–916
Merino S, Moreno J, Sanz JJ, Arriero E (2000) Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). P Roy Soc B-Biol Sci 267:2507–2510
Michaud V, Gil P, Kwiatek O, Prome S, Dixon L, Romero L, Le Potier MF, Arias M, Couacy-Hymann E, Roger F, Libeau G, Albina E (2007) Long-term storage at tropical temperature of dried blood filter papers for detection and genotyping of RNA and DNA viruses by direct PCR. J Virol Methods 146:257–265. doi:10.1016/j.jviromet.2007.07.006
Moens MAJ, Valkiūnas G, Paca A, Bonaccorso E, Aguirre N, Pérez-Tris J (2016) Parasite specialization in a unique habitat: hummingbirds as reservoirs of generalist blood parasites of Andean birds. J Anim Ecol. doi:10.1111/1365-2656.12550
Olias P, Wegelin M, Zenker W, Freter S, Gruber AD, Klopfleisch R (2011) Avian malaria deaths in parrots, Europe. Emerg Infect Dis 17(5):950–952. doi:10.3201/eid1705.101618
Owen JC (2011) Collecting, processing, and storing avian blood: a review. J Field Ornithol 82:339–354. doi:10.1111/j.1557-9263.2011.00338.x
Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Glass RI (2009) Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 200(Suppl(5)):S9–S15. doi:10.1086/605025
Perkins SL, Schall J (2002) A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol 88(5):972–978
Ricklefs RE, Fallon SM (2002) Diversification and host switching in avian malaria parasites. P Roy Soc Lond B Bio 269:885–892
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 (12):1572–1574. doi:10.1093/bioinformatics/btg180
Sato Y, Hagihara M, Yamaguchi T, Yukawa M, Murata K (2007). Phylogenetic comparison of Leucocytozoon spp. from wild birds of Japan. J Vet Med Sci 69(1):55–59
Valkiūnas G (2005) Avian malaria parasites and other Haemosporidia. CRC, Boca Raton
Valkiūnas G, Iezhova TA (2004) Detrimental effects of Haemoproteus infections on the survival of biting midge Culicoides impunctatus (Dipteraceratopogonidae). J Parasitol 90:194–196
Valkiūnas G, Iezhova TA, Brooks DR, Hanelt B, Brant SV, Sutherlin ME, Causey D (2004) Additional observations on blood parasites of birds in Costa Rica. J Wildlife Dis 40:555–561
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Sehgal RNM, Bensch S (2008) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401. doi:10.1645/GE-1570.1
Waldeonstöm J, Bensch S, Hasselquist D, Östman Ö (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194
White E, Bennett GN, Williams NA (1979) Avian Haemoproteidae. 11. The haemoproteids of the hummingbird family Trochilidae. Can J Zool 57:908–913
Williamson S (2001) Hummingbirds of North America. Peterson field guide. Houghton Mifflin Company, New York
Yanega GM, Rubega MA (2004) Feeding mechanisms: Hummingbird jaw bends to aid insect capture. Nature 428(6983):615–615
Acknowledgements
We appreciate the collaboration of many people with their institutions who helped make this research possible. The following individuals provided technical and field assistance for this research: J. Snipes, T. Drazenovich, K. Hagadorn, Y. Adedeji, S. Wheeler, E. Walther, L. Dalbeck, H.W. Liu, N. Pedersen, M. Buchalski, S. Wethington, R. Colwell, B. Robinson, S. Wetzlich, A. Engilis, I. Engilis, J. Trochet, R. Sarvani, many hummingbird banders and volunteers, the UC Davis School of Veterinary Medicine Teaching Hospital, the UC Davis Museum of Wildlife and Fish Biology, California Animal Health and Food Safety Laboratory (CAHFS), California Department of Health Services, California Department of Fish and Wildlife, Lindsay Wildlife Hospital (Walnut Creek, CA), and several wildlife rehabilitation facilities. This work is presented in memory of Loreto Godoy, who provided help to get this project started. Funding for this work was provided from the US Fish and Wildlife Service Avian Disease Ecology Grant (H.B.E. and L.A.T.), a Students Training in Advanced Research fellowship from UC Davis School of Veterinary Medicine (S.B.), and a UC Davis Academic Senate research grant (H.B.E.). We also thank Dr. Gediminas Valkiūnas for insightful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bradshaw, A.C., Tell, L.A., Ernest, H.B. et al. Detection and prevalence of Haemoproteus archilochus (Haemosporida, Haemoproteidae) in two species of California hummingbirds. Parasitol Res 116, 1879–1885 (2017). https://doi.org/10.1007/s00436-017-5463-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5463-5